Beyond Rapid Relief by Sci-chem International Pty Ltd Beyond Rapid Relief
Beyond Rapid Relief by
Drug Labeling and Warnings
Beyond Rapid Relief by is a Otc medication manufactured, distributed, or labeled by Sci-chem International Pty Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
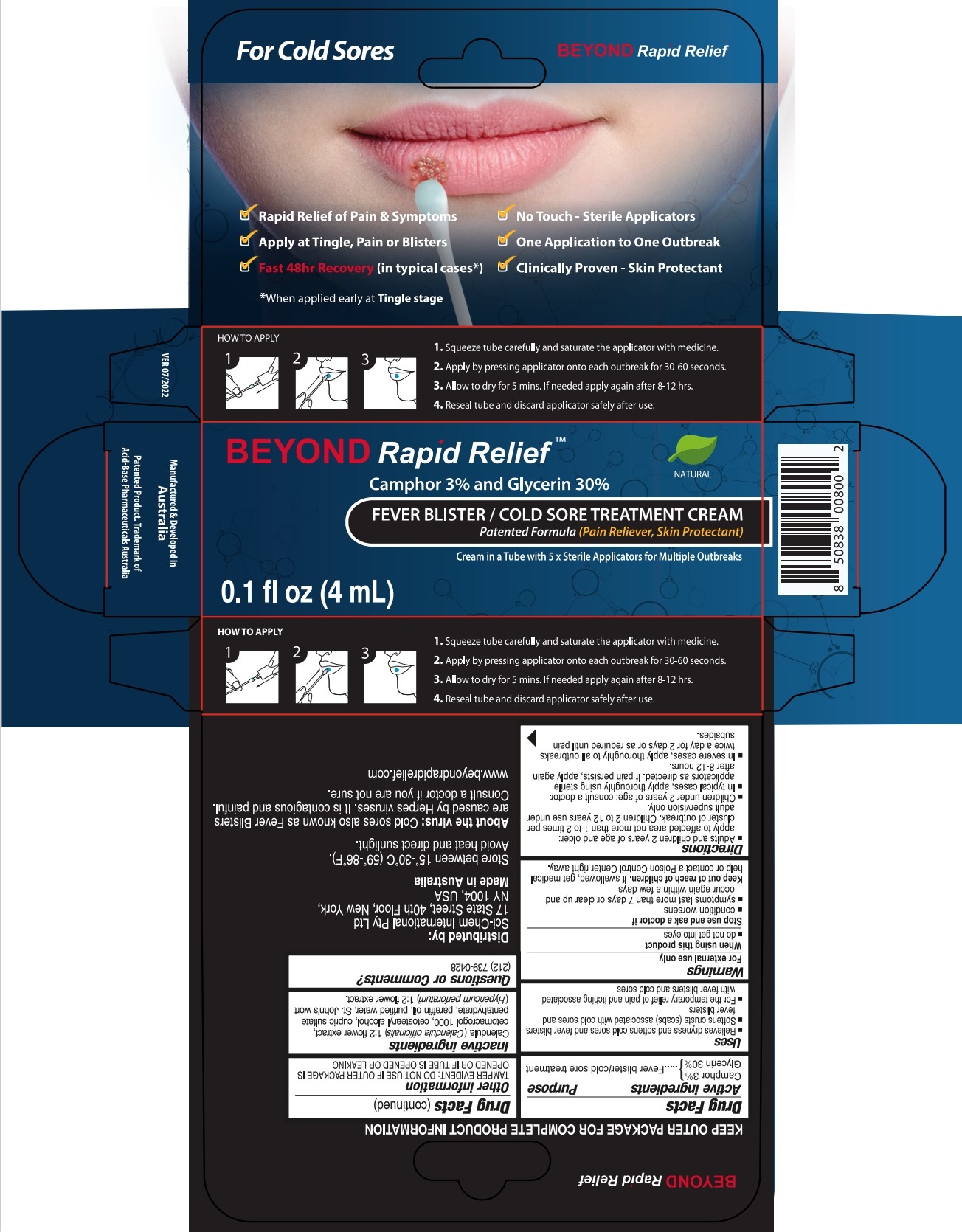
BEYOND RAPID RELIEF- camphor (natural), glycerin cream
Sci-chem International Pty Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Beyond Rapid Relief
Uses
- Relieves dryness and softens cold sores and fever blisters
- Softens crusts (scabs) associated with cold sores and fever blisters.
- For the temporary relief of pain and itching associated with fever blisters and cold sores.
Warnings
For external use only
Directions
- Adults and children 2 years of age and older: apply to affected area not more than 1 to 2 times per cluster of outbreak. Children 2 to 12 years use under adult supervision only.
- Children under 2 years of age: consult a doctor.
- In typical cases, apply thoroughly using sterile applicators as directed. If pain persists, apply again after 8-12 hours.
- In severe cases, apply thoroughly to all outbreaks twice a day for 2 days or as required until pain subsides.
Other information
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR IF TUBE IS OPENED OR LEAKING
| BEYOND RAPID RELIEF
camphor (natural), glycerin cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sci-chem International Pty Ltd (753878391) |
| Registrant - Sci-chem International Pty Ltd (753878391) |
Revised: 5/2023
Document Id: fbbda876-e611-0868-e053-6294a90adaf2
Set id: e6f998da-65b2-41cd-9783-73514d7aed5f
Version: 3
Effective Time: 20230515