FLUTICASONE PROPIONATE spray, metered
Fluticasone Propionate by
Drug Labeling and Warnings
Fluticasone Propionate by is a Prescription medication manufactured, distributed, or labeled by Unit Dose Services. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Fluticasone propionate, the active component of Fluticasone Propionate Nasal Spray, USP, is a synthetic corticosteroid having the chemical name -(fluoromethyl) 6α,9-difluoro-11β-17-dihydroxy- 16α-methyl-3-oxoandrosta-1,4- diene-17β-carbothioate, 17-propionate and the following chemical structure: S
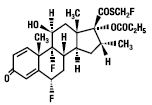
Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C H F O S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol. 253135
Fluticasone Propionate Nasal Spray, USP, 50 mcg is an aqueous suspension of microfine fluticasone propionate for topical administration to the nasal mucosa by means of a metering, atomizing spray pump. Fluticasone Propionate Nasal Spray, USP, also contains benzalkonium chloride (0.02% w/w), carboxymethylcellulose sodium,dextrose, microcrystalline cellulose, phenylethyl alchol (0.25% w/w), polysorbate 80, and purified water and has a pH between 5.8 and 6.8.
It is necessary to prime the pump before first use or after a period of non-use (1 week or more). After initial priming (6 actuations), each actuation delivers 50 mcg of fluticasone propionate in 100 mg of formulation through the nasal adapter. Each 16-g bottle of Fluticasone Propionate Nasal Spray, USP, provides 120 metered sprays. After 120 metered sprays, the amount of fluticasone propionate delivered per actuation may not be consistent and the unit should be discarded.
-
CLINICAL PHARMACOLOGY
Mechanism of Action:
Fluticasone propionate is a synthetic, trifluorinated corticosteroid with anti-inflammatory activity. In vitro dose response studies on a cloned human glucocorticoid receptor system involving binding and gene expression afforded 50% responses at 1.25 and 0.17 nM concentrations, respectively. Fluticasone propionate was 3-fold to 5-fold more potent than dexamethasone in these assays. Data from the McKenzie vasoconstrictor assay in man also support its potent glucocorticoid activity.
In preclinical studies, fluticasone propionate revealed progesterone-like activity similar to the natural hormone. However, the clinical significance of these findings in relation to the low plasma levels (see ) is not known. Pharmacokinetics
The precise mechanism through which fluticasone propionate affects allergic rhinitis symptoms is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. In 7 trials in adults, Fluticasone Propionate Nasal Spray,USP, has decreased nasal mucosal eosinophils in 66% (35% for placebo) of patients and basophils in 39% (28% for placebo) of patients. The direct relationship of these finding to long-term symptom relief is not known.
Fluticasone Propionate Nasal Spray, USP, like other corticosteroids, is an agent that does not have an immediate effect on allergic symptoms. A decrease in nasal symptoms has been noted in some patients 12 hours after initial treatment with Fluticasone Propionate Nasal Spray, USP. Maximum benefit may not be reached for several days. Similarly, when corticosteroids are discontinued, symptoms may not return for several days.
Pharmacokinetics
Absorption
The activity of Fluticasone Propionate Nasal Spray, USP, is due to the parent drug, fluticasone propionate. Indirect calculations indicate that fluticasone propionate delivered by the intranasal route has an absolute bioavailability averaging less than 2%. After intranasal treatment of patients with allergic rhinitis for 3 weeks, fluticasone propionate plasma concentrations were above the level of detection (50 pg/mL) only when recommended doses were exceeded and then only in occasional samples at low plasma levels. Due to the low bioavailability by the intranasal route, the majority of the pharmacokinetic data was obtained via other routes of administration. Studies using oral dosing of radiolabeled drug have demonstrated that fluticasone propionate is highly extracted from plasma and absorption is low. Oral bioavailability is negligible, and the majority of the circulating radioactivity is due to an inactive metabolite.
Distribution
Following intravenous administration, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The volume of distribution averaged 4.2 L/kg.
The percentage of fluticasone propionate bound to human plasma proteins averaged 91% with no obvious concentration relationship. Fluticasone propionate is weakly and reversibly bound to erythrocytes and freely equilibrates between erythrocytes and plasma. Fluticasone propionate is not significantly bound to human transcortin.
Metabolism
The total blood clearance of fluticasone propionate is high (average, 1,093 mL/min), with renal clearance accounting for less than 0.02% of the total. The only circulating metabolite detected in man is the 17β-carboxylic acid derivative of fluticasone propionate, which is formed through the cytochrome P450 3A4 pathway. This inactive metabolite had less affinity (approximately 1/2,000) than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
Elimination
Following intravenous dosing, fluticasone propionate showed polyexponential kinetics and had a terminal elimination half-life of approximately 7.8 hours. Less than 5% of a radiolabeled oral dose was excreted in the urine as metabolites, with the remainder excreted in the feces as parent drug and metabolites.
Special Populations
Fluticasone Propionate Nasal Spray, USP, was not studied in any special populations, and no gender-specific pharmacokinetic data have been obtained.
Drug Interactions
Fluticasone propionate is a substrate of cytochrome P450 3A4. Coadministration of fluticasone propionate and the highly potent cytochrome P450 3A4 inhibitor ritonavir is not recommended based upon a multiple- dose, crossover drug interaction study in 18 healthy subjects. Fluticasone propionate aqueous nasal spray (200 mcg once daily) was coadministered for 7 days with ritonavir (100 mg twice daily). Plasma fluticasone propionate concentrations following fluticasone propionate aqueous nasal spray alone were undetectable (<10 pg/mL) in most subjects, and when concentrations were detectable, peak levels (C ) averaged 11.9 pg/mL (range, 10.8 to 14.1 pg/mL) and AUC averaged 8.43 pghr/mL (range, 4.2 to 18.8 pghr/mL). Fluticasone propionate C and AUC increased to 318 pg/mL (range, 110 to 648 pg/mL) and 3,102.6 pghr/mL (range, 1,207.1 to 5,662.0 pghr/mL), respectively, after coadministration of ritonavir with fluticasone propionate aqueous nasal spray. This significant increase in plasma fluticasone propionate exposure resulted in a significant decrease (86%) in plasma cortisol area under the plasma concentration versus time curve (AUC). max(0-t)max(0-t)
Caution should be exercised when other potent cytochrome P450 3A4 inhibitors are coadministered with fluticasone propionate. In a drug interaction study, coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in increased fluticasone propionate exposure and reduced plasma cortisol AUC, but had no effect on urinary excretion of cortisol.
In another multiple-dose drug interaction study, coadministration of orally inhaled fluticasone propionate (500 mcg twice daily) and erythromycin (333 mg 3 times daily) did not affect fluticasone propionate pharmacokinetics.
Pharmacodynamics
In a trial to evaluate the potential systemic and topical effects of Fluticasone Propionate Nasal Spray, USP, on allergic rhinitis symptoms, the benefits of comparable drug blood levels produced by Fluticasone Propionate Nasal Spray, USP, and oral fluticasone propionate were compared. The dosages used were 200 mcg of Fluticasone Propionate Nasal Spray, USP, the nasal spray vehicle (plus oral placebo), and 5 and 10 mg of oral fluticasone propionate (plus nasal spray vehicle) per day for 14 days. Plasma levels were undetectable in the majority of patients after intranasal dosing, but present at low levels in the majority after oral dosing. Fluticasone Propionate Nasal Spray, USP, was significantly more effective in reducing symptoms of allergic rhinitis than either the oral fluticasone propionate or the nasal vehicle. This trial demonstrated that the therapeutic effect of Fluticasone Propionate Nasal Spray, USP, can be attributed to the topical effects of fluticasone propionate.
In another trial, the potential systemic effects of Fluticasone Propionate Nasal Spray, USP, on the hypothalamic-pituitaryadrenal (HPA) axis were also studied in allergic patients. Fluticasone Propionate Nasal Spray, USP, given as 200 mcg once daily or 400 mcg twice daily was compared with placebo or oral prednisone 7.5 or 15 mg given in the morning. Fluticasone Propionate Nasal Spray, USP, at either dosage for 4 weeks did not affect the adrenal response to 6-hour cosyntropin stimulation, while both dosages of oral prednisone significantly reduced the response to cosyntropin.
-
CLINICAL TRIALS
A total of 13 randomized, double-blind, parallel-group, multicenter, vehicle placebo-controlled clinical trials were conducted in the United States in adults and pediatric patients (4 years of age and older) to investigate regular use of Fluticasone Propionate Nasal Spray, USP, in patients with seasonal or perennial allergic rhinitis. The trials included 2,633 adults (1,439 men and 1,194 women) with a mean age of 37 (range, 18 to 79 years). A total of 440 adolescents (405 boys and 35 girls), mean age of 14 (range, 12 to 17 years), and 500 children (325 boys and 175 girls), mean age of 9 (range, 4 to 11 years) were also studied. The overall racial distribution was 89% white, 4% black, and 7% other. These trials evaluated the total nasal symptom scores (TNSS) that included rhinorrhea, nasal obstruction, sneezing, and nasal itching in known allergic patients who were treated for 2 to 24 weeks. Subjects treated with Fluticasone Propionate Nasal Spray, USP exhibited significantly greater decreases in TNSS than vehicle placebo-treated patients. Nasal mucosal basophils and eosinophils were also reduced at the end of treatment in adult studies; however, the clinical significance of this decrease is not known.
There were no significant differences between fluticasone propionate regimens whether administered as a single daily dose of 200 mcg (two 50-mcg sprays in each nostril) or as 100 mcg (one 50-mcg spray in each nostril) twice daily in 6 clinical trials. A clear dose response could not be identified in clinical trials. In 1 trial, 200 mcg/day was slightly more effective than 50 mcg/day during the first few days of treatment; thereafter, no difference was seen.
Two randomized, double-blind, parallelgroup, multicenter, vehicle placebo-controlled 28-day trials were conducted in the United States in 732 patients (243 given Fluticasone Propionate Nasal Spray, USP) 12 years of age and older to investigate “as-needed” use of Fluticasone Propionate Nasal Spray, USP (200 mcg) in patients with seasonal allergic rhinitis. Patients were instructed to take the study medication only on days when they thought they needed the medication for symptom control, not to exceed 2 sprays per nostril on any day, and not more than once daily. “Asneeded” use was prospectively defined as average use of study medication no more than 75% of study days. Average use of study medications was 57% to 70% of days for all treatment arms. The studies demonstrated significantly greater reduction in TNSS (sum of nasal congestion, rhinorrhea, sneezing, and nasal itching) with Fluticasone Propionate Nasal Spray, USP, 200 mcg compared to placebo. The relative difference in efficacy with as-needed use as compared to regularly administered doses was not studied.
Three randomized, double-blind, parallel- group, vehicle placebo-controlled trials were conducted in 1,191 patients to investigate regular use of Fluticasone Propionate Nasal Spray, USP, in patients with perennial nonallergic rhinitis. These trials evaluated the patient-rated TNSS (nasal obstruction, postnasal drip, rhinorrhea) in patients treated for 28 days of doubleblind therapy and in 1 of the 3 trials for 6 months of open-label treatment. Two of these trials demonstrated that patients treated with Fluticasone Propionate Nasal Spray, USP, at a dosage of 100 mcg twice daily exhibited statistically significant decreases in TNSS compared with patients treated with vehicle.
Individualization of Dosage
Patients should use Fluticasone Propionate Nasal Spray, USP, at regular intervals for optimal effect.
Adult patients may be started on a 200-mcg once-daily regimen (two 50-mcg sprays in each nostril once daily). An alternative 200-mcg/day dosage regimen can be given as 100 mcg twice daily (one 50-mcg spray in each nostril twice daily).
Individual patients will experience a variable time to onset and different degree of symptom relief. In 4 randomized, doubleblind, vehicle placebo-controlled, parallel- group allergic rhinitis studies and 2 studies of patients in an outdoor “park” setting (park studies), a decrease in nasal symptoms in treated subjects compared to placebo was shown to occur as soon as 12 hours after treatment with a 200-mcg dose of Fluticasone Propionate Nasal Spray, USP. Maximum effect may take several days. Regular-use patients who have responded may be able to be maintained (after 4 to 7 days) on 100 mcg/day (1 spray in each nostril once daily).
Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of Fluticasone Propionate Nasal Spray, USP (not to exceed 200 mcg daily) effective for symptom control (see ). Greater symptom control may be achieved with scheduled regular use. Efficacy of asneeded use of Fluticasone Propionate Nasal Spray, USP, has not been studied in pediatric patients under 12 years of age with seasonal allergic rhinitis, or patients with perennial allergic or nonallergic rhinitis. CLINICAL TRIALS
Pediatric patients (4 years of age and older) should be started with 100 mcg (1 spray in each nostril once daily). Treatment with 200 mcg (2 sprays in each nostril once daily or 1 spray in each nostril twice daily) should be reserved for pediatric patients not adequately responding to 100 mcg daily. Once adequate control is achieved, the dosage should be decreased to 100 mcg (1 spray in each nostril) daily.
Maximum total daily doses should not exceed 2 sprays in each nostril (total dose, 200 mcg/day). There is no evidence that exceeding the recommended dose is more effective.
-
INDICATIONS AND USAGE
Fluticasone Propionate Nasal Spray, USP, is indicated for the management of the nasal symptoms of seasonal and perennial allergic and nonallergic rhinitis in adults and pediatric patients 4 years of age and older.
Safety and effectiveness of Fluticasone Propionate Nasal Spray, USP, in children below 4 years of age have not been adequately established.
- CONTRAINDICATIONS
-
WARNINGS
The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency, and in addition some patients may experience symptoms of withdrawal, e.g., joint and/or muscular pain, lassitude, and depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions requiring long-term systemic corticosteroid treatment, too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
The concomitant use of intranasal corticosteroids with other inhaled corticosteroids could increase the risk of signs or symptoms of hypercorticism and/or suppression of the HPA axis.
A drug interaction study in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations (see and ). During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects. CLINICAL PHARMACOLOGY: Drug InteractionsPRECAUTIONS: Drug Interactions
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Avoid spraying in eyes.
-
PRECAUTIONS
General
Intranasal corticosteroids may cause a reduction in growth velocity when administered to pediatric patients (see ). Rarely, immediate hypersensitivity reactions or contact dermatitis may occur after the administration of Fluticasone Propionate Nasal Spray, USP. Rare instances of wheezing, nasal septum perforation, cataracts, glaucoma, and increased intraocular pressure have been reported following the intranasal application of corticosteroids, including fluticasone propionate. PRECAUTIONS: Pediatric use
Use of excessive doses of corticosteroids may lead to signs or symptoms of hypercorticism and/or suppression of HPA function.
Although systemic effects have been minimal with recommended doses of Fluticasone Propionate Nasal Spray, USP, potential risk increases with larger doses. Therefore, larger than recommended doses of Fluticasone Propionate Nasal Spray, USP, should be avoided.
When used at higher than recommended doses or in rare individuals at recommended doses, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, the dosage of Fluticasone Propionate Nasal Spray, USP, should be discontinued slowly consistent with accepted procedures for discontinuing oral corticosteroid therapy.
In clinical studies with fluticasone propionate administered intranasally, the development of localized infections of the nose and pharynx with albicans has occurred only rarely. When such an infection develops, it may require treatment with appropriate local therapy and discontinuation of treatment with Fluticasone Propionate Nasal Spray, USP. Patients using Fluticasone Propionate Nasal Spray, USP, over several months or longer should be examined periodically for evidence of Candida infection or other signs of adverse effects on the nasal mucosa. Candida
Intranasal corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculous infections of the respiratory tract; untreated local or systemic fungal or bacterial infections; systemic viral or parasitic infections; or ocular herpes simplex.
Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred.
Information for patients
Patients being treated with Fluticasone Propionate Nasal Spray, USP, should receive the following information and instructions. This information is intended to aid them in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Patients should be warned to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay.
Patients should use Fluticasone Propionate Nasal Spray, USP, at regular intervals for optimal effect. Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of 200 mcg once daily effective for symptom control (see ). CLINICAL TRIALS
A decrease in nasal symptoms may occur as soon as 12 hours after starting therapy with Fluticasone Propionate Nasal Spray, USP. Results in several clinical trials indicate statistically significant improvement within the first day or two of treatment; however, the full benefit of Fluticasone Propionate Nasal Spray, USP, may not be achieved until treatment has been administered for several days. The patient should not increase the prescribed dosage but should contact the physician if symptoms do not improve or if the condition worsens.
For the proper use of Fluticasone Propionate Nasal Spray, USP, and to attain maximum improvement, the patient should read and follow carefully the patient’s instructions accompanying the product.
Drug interactions
Fluticasone propionate is a substrate of cytochrome P450 3A4. A drug interaction study with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations (see ). During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing syndrome and adrenal suppression. Therefore, coadministration of fluticasone propionate and ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects. CLINICAL PHARMACOLOGY: Drug Interactions
In a placebo-controlled, crossover study in 8 healthy volunteers, coadministration of a single dose of orally inhaled fluticasone propionate (1,000 mcg; 5 times the maximum daily intranasal dose) with multiple doses of ketoconazole (200 mg) to steady state resulted in increased plasma fluticasone propionate exposure, a reduction in plasma cortisol AUC, and no effect on urinary excretion of cortisol. Caution should be exercised when Fluticasone Propionate Nasal Spray, USP, is coadministered with ketoconazole and other known potent cytochrome P450 3A4 inhibitors.
Carcinogenesis, mutagenesis, impairment of fertility
Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 20 times the maximum recommended daily intranasal dose in adults and approximately 10 times the maximum recommended daily intranasal dose in children on a mcg/m basis) for 78 weeks or in rats at inhalation doses up to 57 mcg/kg (approximately 2 times the maximum recommended daily intranasal dose in adults and approximately equivalent to the maximum recommended daily intranasal dose in children on a mcg/m basis) for 104 weeks. 22
Fluticasone propionate did not induce gene mutation in prokaryotic or eukaryotic cells in vitro. No significant clastogenic effect was seen in cultured human peripheral lymphocytes in vitro or in the mouse micronucleus test.
No evidence of impairment of fertility was observed in reproductive studies conducted in male and female rats at subcutaneous doses up to 50 mcg/kg (approximately 2 times the maximum recommended daily intranasal dose in adults on a mcg/m basis). Prostate weight was significantly reduced at a subcutaneous dose of 50 mcg/kg. 2
Pregnancy
Teratogenic effects
Pregnancy Category C. Subcutaneous studies in the mouse and rat at 45 and 100 mcg/kg, respectively (approximately equivalent to and 4 times, respectively, the maximum recommended daily intranasal dose in adults on a mcg/m basis) revealed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification. 2
In the rabbit, fetal weight reduction and cleft palate were observed at a subcutaneous dose of 4 mcg/kg (less than the maximum recommended daily intranasal dose in adults on a mcg/m basis). 2
However, no teratogenic effects were reported at oral doses up to 300 mcg/kg (approximately 25 times the maximum recommended daily intranasal dose in adults on a mcg/m basis), of fluticasone propionate to the rabbit. No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration (see ). 2CLINICAL PHARMACOLOGY
Fluticasone propionate crossed the placenta following oral administration of 100 mcg/kg to rats and 300 mcg/kg to rabbits (approximately 4 and 25 times, respectively, the maximum recommended daily intranasal dose in adults on a mcg/m basis). 2
There are no adequate and well-controlled studies in pregnant women. Fluticasone propionate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Nursing mothers
It is not known whether fluticasone propionate is excreted in human breast milk. However, other corticosteroids have been detected in human milk. Subcutaneous administration to lactating rats of 10 mcg/kg of tritiated fluticasone propionate (less than the maximum recommended daily intranasal dose in adults on a mcg/m basis) resulted in measurable radioactivity in the milk. Since there are no data from controlled trials on the use of intranasal fluticasone propionate by nursing mothers, caution should be exercised when Fluticasone Propionate Nasal Spray, USP, is administered to a nursing woman. 2
Pediatric use
Six hundred fifty (650) patients aged 4 to 11 years and 440 patients aged 12 to 17 years were studied in US clinical trials with Fluticasone Propionate Nasal Spray, USP. The safety and effectiveness of Fluticasone Propionate Nasal Spray, USP, in children below 4 years of age have not been established.
Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including Fluticasone Propionate Nasal Spray, USP, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks/benefits of treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, including Fluticasone Propionate Nasal Spray, USP, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
A 1-year placebo-controlled clinical growth study was conducted in 150 pediatric patients (ages 3 to 9 years) to assess the effect of Fluticasone Propionate Nasal Spray, USP (single daily dose of 200 mcg, the maximum approved dose) on growth velocity. From the primary population of 56 patients receiving Fluticasone Propionate Nasal Spray, USP and 52 receiving placebo, the point estimate for growth velocity with Fluticasone Propionate Nasal Spray, USP was 0.14 cm/year lower than that noted with placebo (95% confidence interval ranging from 0.54 cm/year lower than placebo to 0.27 cm/year higher than placebo). Thus, no statistically significant effect on growth was noted compared to placebo. No evidence of clinically relevant changes in HPA axis function or bone mineral density was observed as assessed by 12-hour urinary cortisol excretion and dual-energy x-ray absorptiometry, respectively.
The potential for Fluticasone Propionate Nasal Spray, USP, to cause growth suppression in susceptible patients or when given at higher doses cannot be ruled out.
Geriatric use
A limited number of patients 65 years of age and older (n = 129) or 75 years of age and older (n = 11) have been treated with Fluticasone Propionate Nasal Spray, USP, in US and non-US clinical trials. While the number of patients is too small to permit separate analysis of efficacy and safety, the adverse reactions reported in this population were similar to those reported by younger patients.
-
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
In controlled US studies, more than 3,300 patients with seasonal allergic, perennial allergic, or perennial nonallergic rhinitis received treatment with intranasal fluticasone propionate. In general, adverse reactions in clinical studies have been primarily associated with irritation of the nasal mucous membranes, and the adverse reactions were reported with approximately the same frequency by patients treated with the vehicle itself. The complaints did not usually interfere with treatment. Less than 2% of patients in clinical trials discontinued because of adverse events; this rate was similar for vehicle placebo and active comparators.
Systemic corticosteroid side effects were not reported during controlled clinical studies up to 6 months’ duration with Fluticasone Propionate Nasal Spray, USP. If recommended doses are exceeded, however, or if individuals are particularly sensitive or taking Fluticasone Propionate Nasal Spray, USP, in conjunction with administration of other corticosteroids, symptoms of hypercorticism, e.g., Cushing syndrome, could occur.
The following incidence of common adverse reactions (>3%, where incidence in fluticasone propionate-treated subjects exceeded placebo) is based upon 7 controlled clinical trials in which 536 patients (57 girls and 108 boys aged 4 to 11 years, 137 female and 234 male adolescents and adults) were treated with Fluticasone Propionate Nasal Spray, USP, 200 mcg once daily over 2 to 4 weeks and 2 controlled clinical trials in which 246 patients (119 female and 127 male adolescents and adults) were treated with Fluticasone Propionate Nasal Spray, USP, 200 mcg once daily over 6 months. Also included in the table are adverse events from 2 studies in which 167 children (45 girls and 122 boys aged 4 to 11 years) were treated with Fluticasone Propionate Nasal Spray, USP, 100 mcg once daily for 2 to 4 weeks.
Overall Adverse Experiences With >3% Incidence on Fluticasone Propionate in Controlled Clinical Trials With Fluticasone Propionate Nasal Spray, USP, in Patients ≥4 Years With Seasonal or Perennial Allergic Rhinitis Adverse Experience Vehicle Placebo
(n=758)
%
Fluticasone Propionate Nasal Spray, USP
100 mcg Once Daily
(n=167)
%
Fluticasone Propionate Nasal Spray, USP
200 mcg Once Daily
(n=782)
%
Headache 14.6 6.6 16.1 Pharyngitis 7.2 6.0 7.8 Epistaxis 5.4 6.0 6.9 Nasal burning/nasal irritation 2.6 2.4 3.2 Nausea/vomitting 2.0 4.8 2.6 Asthma symptoms 2.9 7.2 3.3 Cough 2.8 3.6 3.8 Other adverse events that occurred in ≤3% but ≥1% of patients and that were more common with fluticasone propionate (with uncertain relationship to treatment) included: blood in nasal mucus, runny nose, abdominal pain, diarrhea, fever, flu-like symptoms, aches and pains, dizziness, bronchitis.
Observed During Clinical Practice
Practice: In addition to adverse events reported from clinical trials, the following events have been identified during postapproval use of intranasal fluticasone propionate in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
General
Hypersensitivity reactions, including angioedema, skin rash, edema of the face and tongue, pruritus, urticaria, bronchospasm, wheezing, dyspnea, and anaphylaxis/anaphylactoid reactions, which in rare instances were severe.
Ear, Nose, and Throat
Alteration or loss of sense of taste and/or smell and, rarely, nasal septal perforation, nasal ulcer, sore throat, throat irritation and dryness, cough, hoarseness, and voice changes.
Eye
Dryness and irritation, conjunctivitis, blurred vision, glaucoma, increased intraocular pressure, and cataracts. Cases of growth suppression have been reported for intranasal corticosteroids, including Fluticasone Propionate Nasal Spray, USP (see ). PRECAUTIONS: Pediatric Use
-
OVERDOSAGE
Chronic overdosage may result in signs/symptoms of hypercorticism (see ). Intranasal administration of 2 mg (10 times the recommended dose) of fluticasone propionate twice daily for 7 days to healthy human volunteers was well tolerated. Single oral doses up to 16 mg have been studied in human volunteers with no acute toxic effects reported. Repeat oral doses up to 80 mg daily for 10 days in volunteers and repeat oral doses up to 10 mg daily for 14 days in patients were well tolerated. Adverse reactions were of mild or moderate severity, and incidences were similar in active and placebo treatment groups. Acute overdosage with this dosage form is unlikely since 1 bottle of Fluticasone Propionate Nasal Spray, USP, contains approximately 8 mg of fluticasone propionate. PRECAUTIONS
The oral and subcutaneous median lethal doses in mice and rats were >1,000 mg/kg (>20,000 and >41,000 times, respectively, the maximum recommended daily intranasal dose in adults and >10,000 and >20,000 times, respectively, the maximum recommended daily intranasal dose in children on a mg/m basis). 2
-
DOSAGE AND ADMINISTRATION
Patients should use Fluticasone Propionate Nasal Spray, USP, at regular intervals for optimal effect.
Adults
The recommended starting dosage in is 2 sprays (50 mcg of fluticasone propionate each) in each nostril once daily (total daily dose, 200 mcg). The same dosage divided into 100 mcg given twice daily (e.g., 8 a.m. and 8 p.m.) is also effective. After the first few days, patients may be able to reduce their dosage to 100 mcg (1 spray in each nostril) once daily for maintenance therapy. Some patients (12 years of age and older) with seasonal allergic rhinitis may find as-needed use of 200 mcg once daily effective for symptom control (see ). Greater symptom control may be achieved with scheduled regular use. adultsCLINICAL TRIALS
Adolescents and Children (4 Years of Age and Older)
Patients should be started with 100 mcg (1 spray in each nostril once daily). Patients not adequately responding to 100 mcg may use 200 mcg (2 sprays in each nostril). Once adequate control is achieved, the dosage should be decreased to 100 mcg (1 spray in each nostril) daily.
The maximum total daily dosage should not exceed 2 sprays in each nostril (200 mcg/day). (See ). CLINICAL TRIALS: Individualization of Dosage
Fluticasone Propionate Nasal Spray, USP, is not recommended for children under 4 years of age.
-
PATIENT INSTRUCTIONS FOR USE
Please read this leaflet carefully before you start to take your medicine. It provides a summary of information on your medicine. For further information ask your doctor or pharmacist.
WHAT YOU SHOULD KNOW ABOUT RHINITIS
Rhinitis is a word that means inflammation of the lining of the nose. If you suffer from rhinitis, your nose becomes stuffy and runny. Rhinitis can also make your nose itchy, and you may sneeze a lot. Rhinitis can be caused by allergies to pollen, animals, molds, or other materials-or it may have a nonallergic cause.
WHAT YOU SHOULD KNOW ABOUT FLUTICASONE PROPIONATE NASAL SPRAY, USP
Your doctor has prescribed Fluticasone Propionate Nasal Spray, USP, a medicine that can help treat your rhinitis. Fluticasone Propionate Nasal Spray, USP, contains fluticasone propionate, which is a synthetic corticosteroid. Corticosteroids are natural substances found in the body that help fight inflammation. When you spray Fluticasone Propionate Nasal Spray, USP, into your nose, it helps to reduce the symptoms of allergic reactions and the stuffiness, runniness, itching, and sneezing that can bother you.
THINGS TO REMEMBER ABOUT FLUTICASONE PROPIONATE NASAL SPRAY, USP
1. Shake gently before using.
2. Use your nasal spray as directed by your doctor. The directions are on the pharmacy label.
3. Keep your nasal spray . out of the reach of children
BEFORE USING YOUR NASAL SPRAY
- If you are pregnant (or intending to become pregnant),
- If you are breastfeeding a baby,
- If you are allergic to Fluticasone Propionate Nasal Spray, USP, or any other nasal corticosteroid,
- If you are taking a medicine containing ritonavir (commonly used to treat HIV infection or AIDS).
In some circumstances, this medicine may not be suitable and your doctor may wish to give you a different medicine. Make sure that your doctor knows what other medicines you are taking. TELL YOUR DOCTOR BEFORE STARTING TO TAKE THIS MEDICINE.
USING YOUR NASAL SPRAY
- Follow the instructions shown in the rest of this leaflet. If you have any problems, tell your doctor or pharmacist.
- It is important that you use it as directed by your doctor. The pharmacist¡¦s label will usually tell you what dose to take and how often. If it doesn¡¦t, or you are not sure, ask your doctor or pharmacist.
DOSAGE
- For , the usual starting dose is . Sometimes your doctor may recommend using 1 spray in each nostril twice a day (morning and evening). You should not use more than a total of 2 sprays in each nostril daily. After you have begun to feel better, 1 spray in each nostril daily may be adequate for you.
ADULTS2 sprays in each nostril once daily
For (4 years of age and older), the usual starting dosage is . Sometimes your doctor may recommend using 2 sprays in each nostril daily. Then, after you have begun to feel better, 1 spray in each nostril daily may be adequate for you. ADOLESCENTS and CHILDREN1 spray in each nostril once daily
- DO NOT use more of your medicine or take it more often than your doctor advises.
- Fluticasone Propionate Nasal Spray, USP, may begin to work within 12 hours of the first dose, but it takes several days of regular use to reach its greatest effect. It is important that you use Fluticasone Propionate Nasal Spray, USP, as prescribed by your doctor. Best results will be obtained by using the spray on a regular basis. If symptoms disappear, contact your doctor for further instructions.
- If you also have itchy, watery eyes, you should tell your doctor. You may be given an additional medicine to treat your eyes. Be careful not to confuse them, particularly if the second medicine is an eye drop.
- If you miss a dose, just take your regularly scheduled next dose when it is due. DO NOT DOUBLE the dose.
HOW TO USE YOUR NASAL SPRAY
Read the complete instructions carefully and use only as directed.
BEFORE USING
1. Shake the bottle gently and then remove the cap (Figure 1).
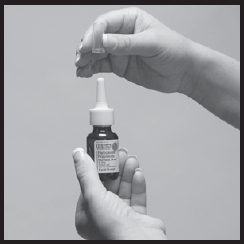
Figure 1
2. It is necessary to prime the pump into the air the first time it is used, or when you have not used it for a week or more. To prime the pump, hold the bottle as shown with the nasal applicator pointing away from you and with your forefinger and middle finger on either side of the nasal applicator and your thumb underneath the bottle. When you prime the pump for the first time, press down and release the pump 6 times (Figure 2). The pump is now ready for use. If the pump is not used for 7 days, prime until a fine spray appears.
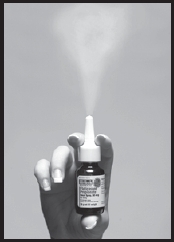
Figure 2
USING THE SPRAY
3. Blow your nose to clear your nostrils
4. Close one nostril. Tilt your head forward slightly and, keeping the bottle upright, carefully insert the nasal applicator into the other nostril (Figure 3).

Figure 3
5. Start to breathe in through your nose, and WHILE BREATHING IN press firmly and quickly down once on the applicator to release the spray. To get a full actuation, use your forefinger and middle finger to spray while supporting the base of the bottle with your thumb. Avoid spraying in eyes. Breathe gently inwards through the nostril (Figure 4).
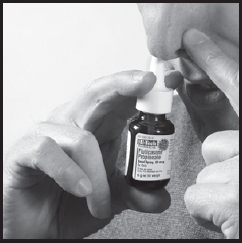
Figure 4
6. Breathe out through your mouth.
7. If a second spray is required in that nostril, repeat steps 4 through 6.
8. Repeat steps 4 through 7 in the other nostril.
9. Wipe the nasal applicator with a clean tissue and replace the cap (Figure 5).
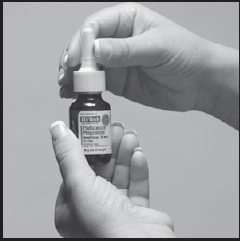
Figure 5
10. Do not use this bottle for more than the labeled number of sprays even though the bottle is not completely empty. Before you throw the bottle away, you should consult your doctor to see if a refill is needed. Do not take extra doses or stop taking Fluticasone Propionate Nasal Spray, USP, without consulting your doctor.
CLEANING
Your nasal spray should be cleaned at least once a week. To do this:
1. Remove the cap and then gently pull upwards to free the nasal applicator.
2. Wash the applicator and cap under warm tap water. Allow to dry at room temperature, then place the applicator and cap back on the bottle.
3. If the nasal applicator becomes blocked, it can be removed as above and left to soak in warm water. Rinse with cold tap water, dry, and refit. Do not try to unblock the nasal applicator by inserting a pin or other sharp object.
STORING YOUR NASAL SPRAY
- Keep your Fluticasone Propionate Nasal Spray, USP, . out of the reach of children
- Avoid spraying in eyes.
- Store between 4° and 30°C (39°F and 86°F).
- Do not use your Fluticasone Propionate Nasal Spray, USP, after the expiration date shown on the label and box.
REMEMBER: This medicine has been prescribed for you by your doctor. DO NOT give this medicine to anyone else.
FURTHER INFORMATION
The leaflet does not contain the complete information about your medicine. . If you have any questions, or are not sure about something, then you should ask your doctor or pharmacist
You may want to read this leaflet again. Please DO NOT THROW IT AWAY until you have finished your medicine.
To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
Hi-Tech Pharmacal Co., Inc.
Amityville, N.Y. 11701
Made in U.S.A.
Rev. 700:05 8/11
- FLUTICASONE PROPIONATE SPRAY, METERED
-
INGREDIENTS AND APPEARANCE
FLUTICASONE PROPIONATE
fluticasone propionate spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50436-9992(NDC:50383-700) Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (FLUTICASONE - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 50 ug in 0.1 g Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) DEXTROSE (UNII: IY9XDZ35W2) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50436-9992-1 16 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077570 01/16/2008 Labeler - Unit Dose Services (831995316) Registrant - Unit Dose Services (831995316) Establishment Name Address ID/FEI Business Operations Unit Dose Services 831995316 REPACK(50436-9992)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
