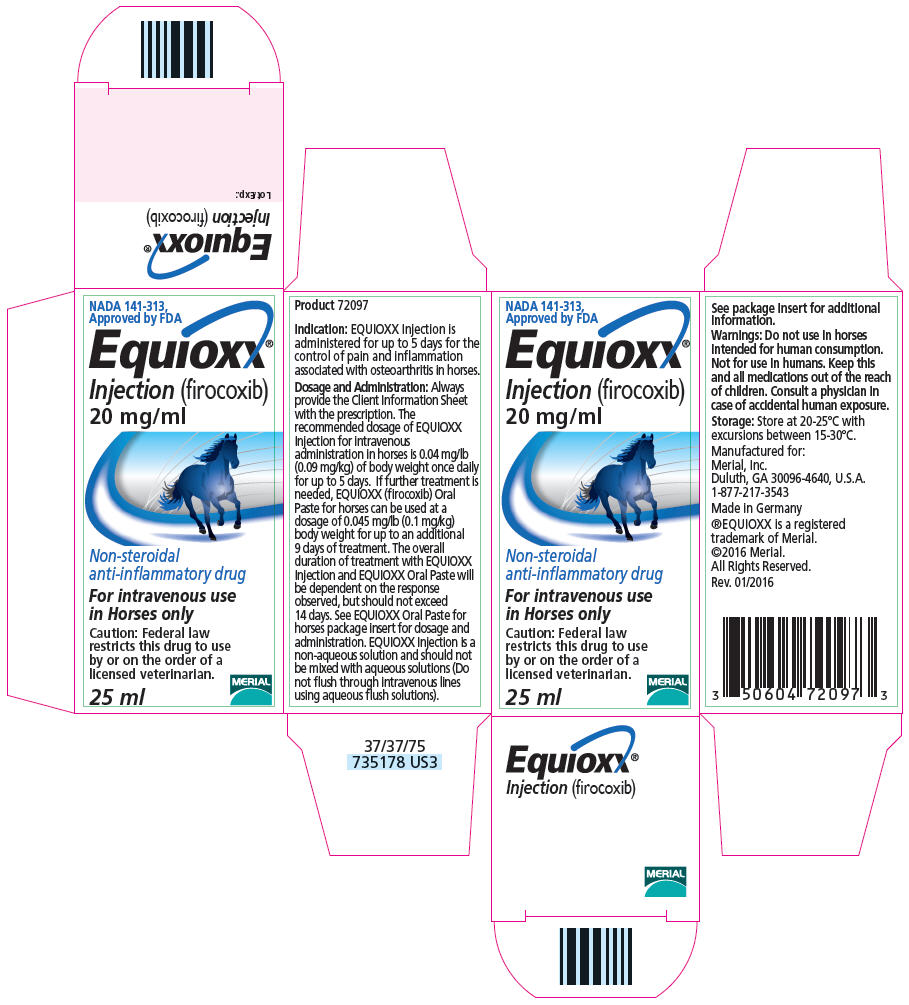
EQUIOXX- firocoxib injection, solution
EQUIOXX by
Drug Labeling and Warnings
EQUIOXX by is a Animal medication manufactured, distributed, or labeled by Merial, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
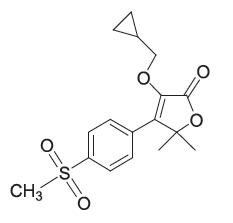
Description: EQUIOXX (firocoxib) belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs (NSAID). Firocoxib is a white crystalline compound described chemically as 3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl)phenyl)-5, 5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4. The structural formula is shown below:
EQUIOXX Injection is a colorless to pale yellow solution. Each mL of EQUIOXX Injection for Horses contains 20 mg of firocoxib as a free base, 550 mg of polyethylene glycol (PEG 400) and 600 mg of glycerol formal.
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Dosage and Administration: Always provide the Client Information Sheet with the prescription. The recommended dosage of EQUIOXX Injection for intravenous administration in horses is 0.04 mg/lb (0.09 mg/kg) of body weight once daily for up to 5 days. If further treatment is needed, EQUIOXX (firocoxib) Oral Paste for horses can be used at a dosage of 0.045 mg/lb (0.1 mg/kg) body weight for up to an additional 9 days of treatment. The overall duration of treatment with EQUIOXX Injection and EQUIOXX Oral Paste will be dependent on the response observed, but should not exceed 14 days. See EQUIOXX Oral Paste for horses package insert for dosage and administration. EQUIOXX Injection is a non-aqueous solution and should not be mixed with aqueous solutions (Do not flush through intravenous lines using aqueous flush solutions).
- CONTRAINDICATIONS
- WARNINGS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
PRECAUTIONS
Precautions: Horses should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data before and periodically during administration of any NSAID. Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription. See Information for Owner or Person Treating Horse section of this package insert.
Treatment with EQUIOXX should be terminated if signs such as inappetance, colic, abnormal feces, or lethargy are observed.
As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Horses that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached or avoided. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforation, concomitant use of EQUIOXX Injection with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided.
The concomitant use of protein bound drugs with EQUIOXX Injection for horses has not been studied in horses. The influence of concomitant drugs that may inhibit the metabolism of firocoxib Injection has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
The safe use of EQUIOXX Injection for horses has not been evaluated in horses less than one year of age, horses used for breeding, or in pregnant or lactating mares.
Consider appropriate washout times when switching from one NSAID to another NSAID or corticosteroid.
-
ADVERSE REACTIONS
Adverse Reactions: The effectiveness of EQUIOXX Injection was established in a biocomparability study demonstrating that EQUIOXX Oral Paste is bioequivalent to EQUIOXX Injection. Thus, additional field studies were not performed to support the effectiveness of EQUIOXX Injection.
In controlled field studies, 127 horses (ages 3 to 37 years) were evaluated for safety when given EQUIOXX® (firocoxib) Oral Paste for Horses at a dose of 0.045 mg/lb (0.1 mg/kg) orally once daily for up to 14 days. The following adverse reactions were observed. Horses may have experienced more than one of the observed adverse reactions during the study.
Adverse Reactions Seen in U.S. Field Studies with EQUIOXX Oral Paste Adverse Reactions EQUIOXX®
n=127Active Control
n=125Abdominal pain 0 1 Diarrhea 2 0 Excitation 1 0 Lethargy 0 1 Loose stool 1 0 Polydipsia 0 1 Urticaria 0 1 EQUIOXX Oral Paste was safely used concomitantly with other therapies, including vaccines, anthelmintics, and antibiotics, during the field studies.
The material safety data sheet (MSDS) contains more detailed occupational safety information. To obtain a material safety data sheet, please call 1-877-217-3543.
-
INFORMATION FOR OWNERS/CAREGIVERS
Information for Owner or Person Treating Horse: You should give a Client Information Sheet to the person treating the horse and advise them of the potential for adverse reactions and the clinical signs associated with NSAID intolerance. Adverse reactions may include erosions and ulcers of the gums, tongue, lips and face, weight loss, colic, diarrhea, or icterus. Serious adverse reactions associated with this drug class can occur without warning and, in some situations, result in death. Clients should be advised to discontinue NSAID therapy and contact their veterinarian immediately if any of these signs of intolerance are observed. The majority of patients with drug-related adverse reactions recover when the signs are recognized, drug administration is stopped, and veterinary care is initiated.
-
PHARMACOKINETICS
Clinical Pharmacokinetics/Pharmacodynamics: Based on the comparison data between the intravenous and oral administration, the area under the curve (AUC) for both routes of administration was the same. The average AUC ratio of injectable to the oral product was 103%. The average peak plasma concentration observed one minute following firocoxib intravenous administration was approximately 3.7 fold greater than the observed average peak plasma concentration reached after administration of the oral paste (oral Tmax = 2.02 hours). The average plasma concentrations following IV injection and oral administration were similar by 2 hours post-dose, after which the concentrations proceeded to decline in parallel. The terminal elimination half-life (T ½ el) values were not significantly different (p>0.05), with values ranging from 14.6 to 68.0 hrs (mean = 31.5 hours) for the oral paste and from 12.6 to 66.3 (mean = 33.0 hours) for the intravenous solution.
The major metabolism mechanism of firocoxib in the horse is decyclopropylmethylation followed by glucuronidation of that metabolite. Based upon radiolabel studies, the majority of firocoxib is eliminated in the urine as the glucuronide conjugate of the decyclopropylmethylated metabolite. Despite a high rate of plasma protein binding (98%), firocoxib exhibits a large volume of distribution (mean Vd (ss) = 1652 mL/kg). The drug accumulation occurs with repeated dose administrations and steady state concentrations are achieved beyond 6-8 daily oral doses in the horse. Dose linearity exists from 1×-2× of 0.1 mg/kg/day after oral administration. Little drug amount distributes into blood cells.
Steady-state plasma firocoxib concentrations at 4 and 24 hours post administration were the same following intravenous or oral administration at each dose in the range of 1× to 5×.
-
MECHANISM OF ACTION
Mode of action: Firocoxib is a cyclooxygenase-inhibiting (coxib) class, non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic activity1 in animal models. Based on in vitro horse data, firocoxib is a selective inhibitor of prostaglandin biosynthesis through inhibition of the inducible cyclooxygenase-2 isoenzyme (COX-2)2,3. Firocoxib selectivity for the constitutive isoenzyme, cyclooxygenase-1 (COX-1), is relatively low. However, the clinical significance of these in vitro selectivity findings has not been established.
-
SPL UNCLASSIFIED SECTION
Effectiveness: The effectiveness of EQUIOXX Injection was established in a biocomparability study evaluating EQUIOXX Oral Paste and EQUIOXX Injection. Thus, additional field studies were not performed to support the effectiveness of EQUIOXX Injection. Two hundred fifty-three client-owned horses of various breeds, ranging in age from 2 to 37 years and weighing from 595 to 1638 lbs, were randomly administered EQUIOXX Oral Paste or an active control drug in multi-center field studies. Two hundred forty horses were evaluated for effectiveness and 252 horses were evaluated for safety. Horses were assessed for lameness, pain on manipulation, range of motion, joint swelling, and overall clinical improvement in a non-inferiority evaluation of EQUIOXX Oral Paste compared to an active control. At study's end, 84.4% of horses treated with EQUIOXX Oral Paste were judged improved on veterinarians' clinical assessment, and 73.8% were also rated improved by owners. Horses treated with EQUIOXX Oral Paste showed improvement in veterinarian-assessed lameness, pain on manipulation, range of motion, and joint swelling that was comparable to the active control.
-
SPL UNCLASSIFIED SECTION
Animal Safety: A target animal safety study was conducted to assess the safety of EQUIOXX Injection followed by EQUIOXX Oral Paste in the horse. Thirty-two clinically healthy adult horses received EQUIOXX Injection intravenously once daily for five days at doses of either 0 mg/kg (control group); 0.09 mg/kg (1×); 0.27 mg/kg (3×); or 0.45 mg/kg (5× the recommended dose). This was followed by once daily oral administration of EQUIOXX Oral paste for nine days at doses of either 0 mg/kg (control group); 0.1 mg/kg (1×); 0.3 mg/kg (3×); or 0.5 mg/kg (5× the recommended dose). This sequence (five days of EQUIOXX Injection followed by nine days EQUIOXX Oral Paste, for a total of 14 days) was repeated three times for a total treatment duration of 42 days (3× the recommended treatment duration of 14 days).
Two male 5× horses demonstrated a white focus in the renal cortex which correlated with tubulointerstitial nephropathy microscopically. The presence of tubulointerstitial nephropathy was considered treatment-related.
One horse from the control group and two horses from the 5× group had injection site swellings during treatment. Injection site changes characterized by inflammatory cell influx and rarely tissue necrosis were seen in all study groups including the control group.
There was a dose-dependent increase in the incidence of oral ulcers and erosions.
Elevated hepatic enzymes (GGT or AST) were noted in all study groups at one or more timepoints. One male 5× horse with an elevated GGT value on Day 42 was noted to have tubulointerstitial nephropathy at the time of necropsy. For all horses, these hepatic enzyme elevations generally returned to the reference range by the next time point.
- STORAGE AND HANDLING
- HOW SUPPLIED
-
REFERENCES
- 1 McCann ME, Rickes EL, Hora DF, Cunningham PK et al. In vitro effects and In vivo efficacy of a novel cyclooxygenase-2 inhibitor in cats with lipopolysaccharide-induced pyrexia. Am J Vet Res. 2005 Jul;66 (7):1278-84
- 2 McCann ME, Anderson DR, Brideau C et al. In vitro activity and in vivo efficacy of a novel COX-2 inhibitor in the horse. Proceedings of the Academy of Veterinary Internal Medicine. 2002. Abstract 114, p.789.
- 3 Data on file.
- SPL UNCLASSIFIED SECTION
-
INFORMATION FOR OWNERS/CAREGIVERS
Equioxx®
Injection (firocoxib)Non-steroidal anti-inflammatory drug for intravenous use in horses only.
Information for Horse Owners
Indication: EQUIOXX Injection is administered for up to 5 days for the control of pain and inflammation associated with osteoarthritis in horses. If further treatment is needed, EQUIOXX Oral Paste can be used for up to an additional 9 days of treatment. The overall duration of treatment with EQUIOXX Injection and EQUIOXX Oral Paste will be dependent on the response observed, but should not exceed 14 days.
This summary contains important information about EQUIOXX. You should read this information before you start giving your horse EQUIOXX and review it each time your prescription is refilled. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want to know more about EQUIOXX.
What is EQUIOXX?
EQUIOXX is a veterinary prescription non-steroidal anti-inflammatory drug (NSAID) of the coxib class used to control pain and inflammation associated with osteoarthritis in horses. Osteoarthritis (OA) is a painful condition caused by progressive "wear and tear" of cartilage and other parts of the joints that may result in the following changes or signs in your horse:
- Limping or lameness.
- Decreased activity or exercise (reluctance to stand, walk, trot or run, or difficulty in performing these activities).
- Stiffness or decreased movement of joints.
How to give EQUIOXX to your horse?
EQUIOXX should be given according to your veterinarian's instructions. Do not change the way you give EQUIOXX to your horse without first speaking with your veterinarian. Do not exceed 14 days of treatment. The recommended dosage of EQUIOXX Injection for intravenous administration in horses is 0.04 mg/lb (0.09 mg/kg) of body weight once daily for up to 5 days. If further treatment is needed, EQUIOXX (firocoxib) Oral Paste for horses can be used at a dosage of 0.045 mg/lb (0.1 mg/kg) bodyweight for up to an additional 9 days of treatment. The overall duration of treatment with EQUIOXX Injection and EQUIOXX Oral Paste will be dependent on the response observed, but should not exceed 14 days.
What kind of results can I expect when my horse is on EQUIOXX for OA?
While EQUIOXX is not a cure for osteoarthritis, it can control the pain and inflammation associated with OA and can improve your horse's mobility.
- Response varies from horse to horse, but improvement can be quite dramatic.
- Improvement can be seen in just a few hours in most horses.
Which horses should not receive EQUIOXX?
Your horse should not be given EQUIOXX if he/she:
- Has an allergic reaction to firocoxib, the active ingredient in EQUIOXX.
- Has previously had an allergic reaction (such as hives, facial or lower limb swelling, or red or itchy skin) to aspirin or other NSAIDs.
- Is presently taking aspirin, phenylbutazone, flunixin meglumine, diclofenac, ketoprofen, or other NSAIDs or corticosteroids.
- The safety of EQUIOXX has not been determined in horses less than one year of age or in breeding horses, pregnant or lactating mares.
EQUIOXX Injection should only be given intravenously to horses.
- EQUIOXX is not for use in horses intended for human food consumption.
- People should not take EQUIOXX. Keep EQUIOXX and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
What to tell/ask your veterinarian before giving EQUIOXX.
Talk to your veterinarian about:
- The signs of OA you have observed in your horse, such as limping or stiffness.
- If any tests, such as X-rays, will be done before EQUIOXX is prescribed.
- How often your horse may need to be examined by your veterinarian.
- The risks and benefits of using EQUIOXX.
Tell your veterinarian if your horse has ever had the following medical problems:
- Any side effects from taking EQUIOXX or other NSAIDs, such as aspirin or phenylbutazone.
- Any kidney disease.
- Any liver disease.
- Any gastrointestinal ulcers.
Tell your veterinarian about:
- Other medical problems or allergies that your horse has now, or has had in the past.
- All medicines that you are giving or plan to give to your horse, including those you can get without a prescription and any dietary supplements.
Tell your veterinarian if you plan to breed your horse, or if your mare is pregnant or nursing a foal.
What are the possible side effects that may occur in my horse during EQUIOXX therapy?
EQUIOXX, like other NSAIDS, may cause some side effects. Serious side effects associated with NSAID therapy in horses can occur with or without warning. The most common side effects associated with EQUIOXX therapy involve the tongue, lips and skin of the mouth and face (erosions and ulcers of the mucosa and skin) and the kidney. Gastrointestinal, kidney and liver problems have also been reported with other NSAIDs. Look for the following side effects that may indicate your horse is having a problem with EQUIOXX or may have another medical problem:
- Sores or ulcers on the tongue and inside of mouth.
- Sores, scabs, redness, or rubbing of the facial skin, particularly around the mouth.
- Change in eating or drinking habits (frequency or amount consumed).
- Change in urination habits (frequency or color).
- Yellowing of gums, skin, or whites of the eyes (jaundice).
- Unexpected weight loss.
- Change in behavior (such as increased or decreased activity level).
It is important to stop therapy and contact your veterinarian if you think your horse has a medical problem or side effect while taking EQUIOXX. If you have additional questions about possible side effects, talk with your veterinarian or call 1-877-217-3543.
Can EQUIOXX be given with other medications?
EQUIOXX should not be given with other NSAIDs (for example, aspirin, phenylbutazone, diclofenac, ketoprofen or flunixin) or systemic corticosteroids (for example, prednisone, cortisone, dexamethasone, or triamcinolone).
Tell your veterinarian about all medications that you have given your horse in the past, and any medications you are planning to give with EQUIOXX. This should include other medicines that you can get without a prescription or any dietary supplements. Your veterinarian may want to check that all of your horse's medicines can be given together.
What do I do in case my horse receives more than the prescribed amount of EQUIOXX?
- Consult your veterinarian if your horse receives more than the prescribed amount of EQUIOXX.
What else should I know about EQUIOXX?
- This sheet provides a summary of information about EQUIOXX paste and general information about NSAIDs. If you have any questions or concerns about EQUIOXX or osteoarthritis pain, talk with your veterinarian.
- As with all prescribed medicines, EQUIOXX should only be given to the horse for which it is prescribed. It should be given to your horse only for the condition for which it is prescribed, at the labeled dose and duration.
- It is important to periodically discuss your horse's response to EQUIOXX. Your veterinarian will determine if your horse is responding as expected and if your horse should continue receiving EQUIOXX.
Manufactured for:
Merial, Inc.
Duluth, GA 30096-4640, U.S.A.
1-877-217-3543Made in Germany
NADA 141-313, Approved by FDA
®EQUIOXX is a registered trademark of Merial.
©2016 Merial. All Rights Reserved.
Rev. 01/2016
723064 US3
- PRINCIPAL DISPLAY PANEL - 25 ml Vial Carton
-
INGREDIENTS AND APPEARANCE
EQUIOXX
firocoxib injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50604-7209 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength firocoxib (UNII: Y6V2W4S4WT) (firocoxib - UNII:Y6V2W4S4WT) firocoxib 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Polyethylene Glycol 400 (UNII: B697894SGQ) glycerol formal (UNII: 3L7GR2604E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50604-7209-1 1 in 1 CARTON 1 25 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141313 09/20/2010 Labeler - Merial, Inc. (799641006)
Trademark Results [EQUIOXX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EQUIOXX 79031787 3298365 Live/Registered |
Merial, Inc. 2006-06-10 |
 EQUIOXX 76274709 2926131 Dead/Cancelled |
MERIAL LIMITED 2001-06-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.