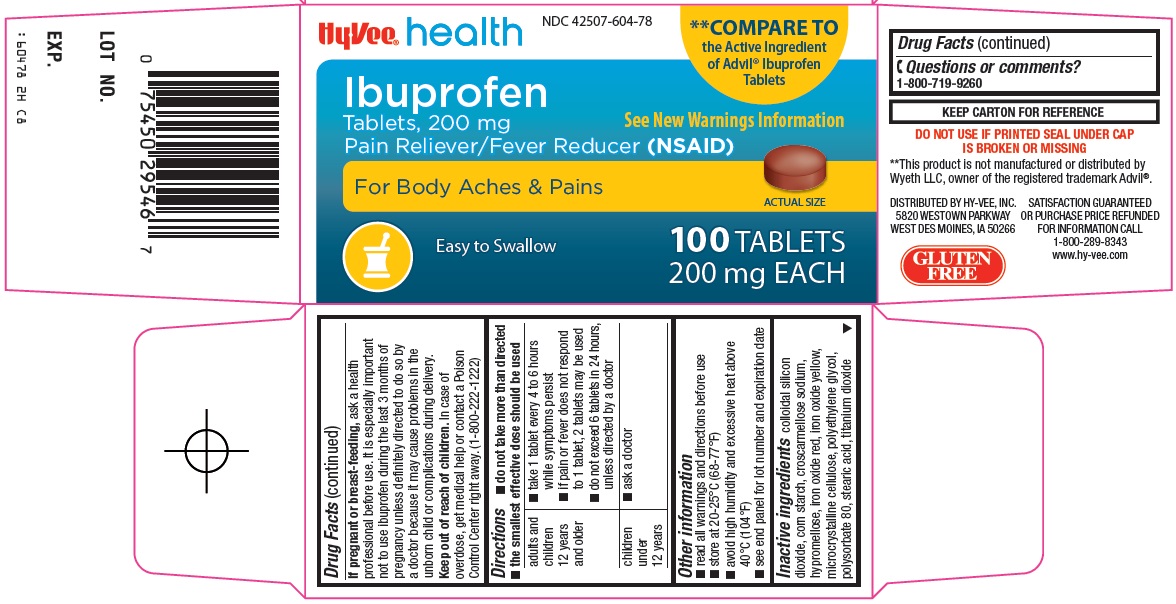
Hy-Vee, Inc. Ibuprofen Tablets, 200 mg Drug Facts
ibuprofen by
Drug Labeling and Warnings
ibuprofen by is a Otc medication manufactured, distributed, or labeled by HyVee Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
IBUPROFEN- ibuprofen tablet, film coated
HyVee Inc
----------
Hy-Vee, Inc. Ibuprofen Tablets, 200 mg Drug Facts
Uses
- temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- minor pain of arthritis
- toothache
- backache
- the common cold
- menstrual cramps
- temporarily reduces fever
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chances are higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- you have problems or serious side effects from taking pain relievers or fever reducers
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- under a doctor’s care for any serious condition
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
Directions
- do not take more than directed
- the smallest effective dose should be used
|
adults and children 12 years and older |
|
|
children under 12 years |
|
Other information
- read all warnings and directions before use
- store at 20-25°C (68-77°F)
- avoid high humidity and excessive heat above 40°C (104°F)
- see end panel for lot number and expiration date {For Carton Configuration Only}
| IBUPROFEN
ibuprofen tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - HyVee Inc (006925671) |
Revised: 11/2019
Document Id: 8712c500-eb6d-43cf-bf5a-74a1d4ca2b87
Set id: e7175dc9-db39-4452-a419-85af179de422
Version: 4
Effective Time: 20191121
HyVee Inc