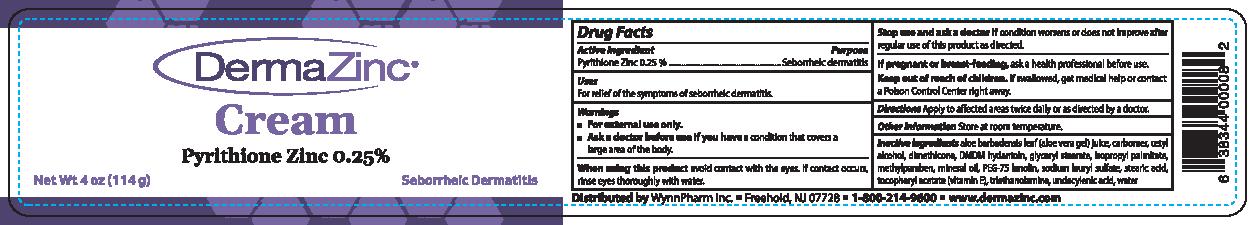
DERMAZINC- pyrithione zinc cream
DermaZinc by
Drug Labeling and Warnings
DermaZinc by is a Otc medication manufactured, distributed, or labeled by WynnPharm Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
WARNINGS
Warnings
■ For external use only.
■ Ask a doctor before use if you have a condition that covers a large area of the body.When using this product avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with
water.
Stop use and ask a doctor if condition worsens or does not improve after regular use of this
product as directed.If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. -
INACTIVE INGREDIENT
Inactive ingredients aloe barbadensis leaf (aloe vera gel) juice, carbomer, cetyl
alcohol, dimethicone, DMDM hydantoin, glyceryl stearate, isopropyl palmitate, methylparaben,
mineral oil, PEG-75 lanolin, sodium 1auryl sulfate, stearic acid, tocopheryl acetate (vitamin E),
triethanolamine,undecylenic acid, water - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMAZINC
pyrithione zinc creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 35324-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 0.25 g in 100 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PEG-75 LANOLIN (UNII: 09179OX7TB) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER 980 (UNII: 4Q93RCW27E) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE 200 (UNII: RGS4T2AS00) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) STEARIC ACID (UNII: 4ELV7Z65AP) METHYLPARABEN (UNII: A2I8C7HI9T) DMDM HYDANTOIN (UNII: BYR0546TOW) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) TROLAMINE (UNII: 9O3K93S3TK) UNDECYLENIC ACID (UNII: K3D86KJ24N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 35324-001-04 114 g in 1 JAR; Type 0: Not a Combination Product 08/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 08/29/2022 Labeler - WynnPharm Inc (620885173) Registrant - WynnPharm Inc (620885173)
Trademark Results [DermaZinc]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DERMAZINC 78844507 3201878 Live/Registered |
Dermalogix Partners, Inc. 2006-03-23 |
 DERMAZINC 75476766 2242858 Dead/Cancelled |
Dermalogix Partners, Inc. 1998-04-29 |
 DERMAZINC 75261328 not registered Dead/Abandoned |
Dermalogix Partners, Inc. 1997-03-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.