These highlights do not include all the information needed to use FAMOTIDINE TABLETS safely and effectively. See full prescribing information for FAMOTIDINE TABLETS. FAMOTIDINE tablets, for oral use Initial U.S. Approval: 1986
Famotidine by
Drug Labeling and Warnings
Famotidine by is a Prescription medication manufactured, distributed, or labeled by REMEDYREPACK INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FAMOTIDINE- famotidine tablet, film coated
REMEDYREPACK INC.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FAMOTIDINE TABLETS safely and effectively. See full prescribing information for FAMOTIDINE TABLETS.
FAMOTIDINE tablets, for oral use Initial U.S. Approval: 1986 INDICATIONS AND USAGEFamotidine tablets are a histamine-2 (H 2) receptor antagonist indicated ( 1): In adult and pediatric patients 40 kg and greater for the treatment of:
In adults for the:
DOSAGE AND ADMINISTRATION
Administration (2.3):
DOSAGE FORMS AND STRENGTHSTablets: 20 mg, 40 mg ( 3) CONTRAINDICATIONSHistory of serious hypersensitivity reactions (e.g., anaphylaxis) to famotidine or other H 2 receptor antagonists. ( 4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSThe most common adverse reactions are: headache, dizziness, constipation, and diarrhea. ( 6.1) To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 8/2019 |
|||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Famotidine tablets are indicated in adult and pediatric patients 40 kg and greater for the treatment of:
- active duodenal ulcer (DU).
- active gastric ulcer (GU).
- symptomatic nonerosive gastroesophageal reflux disease (GERD).
- erosive esophagitis due to GERD, diagnosed by biopsy.
Famotidine tablets are indicated in adults for the:
- treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome, multiple endocrine neoplasias).
- reduction of the risk of duodenal ulcer recurrence.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Table 1 shows the recommended dosage of famotidine 20 mg and 40 mg tablets in adult and pediatric patients weighing 40 kg and greater with normal renal function. The use of famotidine 20 mg and 40 mg tablets is not recommended in pediatric patients weighing less than 40 kg because the lowest available strength (20 mg) exceeds the recommended dose for these patients. Use another famotidine formulation for pediatric patients weighing less than 40 kg.
|
|
||
|
Indication |
Recommended Dosage |
Recommended Duration |
|
Active duodenal ulcer (DU) |
40 mg once daily; or 20 mg twice daily * | |
|
Active gastric ulcer |
40 mg once daily |
Up to 8 weeks ‡ |
|
Symptomatic nonerosive GERD |
20 mg twice daily |
Up to 6 weeks ‡ |
|
Erosive esophagitis diagnosed by endoscopy |
20 mg twice daily; or 40 mg twice daily * |
Up to 12 weeks |
|
Pathological hypersecretory conditions§ |
Starting dosage: 20 mg every 6 hours; adjust dosage to individual patient needs Maximum dosage 160 mg every 6 hours |
As clinically indicated |
|
Reduction of the risk of DU recurrence§ |
20 mg once daily |
1 year ‡ or as clinically indicated |
2.2 Dosage in Renal Impairment
Dosage adjustments of famotidine tablets are recommended for patients with moderate to severe renal impairment (creatinine clearance less than 60 mL/min) [see Use in Specific Populations (8.6)] . Table 2 shows the recommended maximum dosage of famotidine 20 mg or 40 mg tablets for patients with renal impairment, by indication. Use the lowest effective dose. Some dosage adjustments may require switching to other formulations of famotidine (e.g., oral suspension, lower dose tablet).
|
|
||
|
Indication |
Recommended Maximum Dosages |
|
|
Creatinine clearance 30 to 60 mL/minute |
Creatinine clearance less than 30 mL/minute |
|
|
Active duodenal ulcer (DU) |
20 mg once daily; or 40 mg every other day |
20 mg every other day * |
|
Active gastric ulcer |
20 mg once daily; or 40 mg every other day |
20 mg every other day * |
|
Symptomatic nonerosive GERD |
20 mg once daily |
20 mg every other day * |
|
Erosive esophagitis diagnosed by endoscopy† |
20 mg once daily; or 40 mg every other day † | |
|
40 mg once daily † |
20 mg once daily † |
|
|
Pathological hypersecretory conditions‡ |
Avoid use § |
|
|
Reduction of the risk of DU recurrence‡ |
20 mg every other day * |
(see footnote) ¶ |
2.3 Administration Instructions
- Take famotidine tablets once daily before bedtime or twice daily in the morning and before bedtime, as recommended.
- Famotidine tablets may be taken with or without food [see Clinical Pharmacology (12.3)] .
- Famotidine tablets may be given with antacids.
3 DOSAGE FORMS AND STRENGTHS
Famotidine Tablets, USP are available containing 20 mg or 40 mg of famotidine, USP.
- The 20 mg tablets are yellow, film-coated, round, unscored tablets debossed with M over F1 on one side of the tablet and blank on the other side.
- The 40 mg tablets are green, film-coated, round, unscored tablets debossed with M over F2 on one side of the tablet and blank on the other side.
4 CONTRAINDICATIONS
Famotidine tablets are contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis) to famotidine or other histamine-2 (H 2) receptor antagonists.
5 WARNINGS AND PRECAUTIONS
5.1 Central Nervous System Adverse Reactions
Central nervous system (CNS) adverse reactions, including confusion, delirium, hallucinations, disorientation, agitation, seizures, and lethargy, have been reported in elderly patients and patients with moderate and severe renal impairment treated with famotidine tablets. Since famotidine blood levels are higher in patients with renal impairment than in patients with normal renal function, dosage adjustments are recommended in patients with renal impairment [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)] .
5.2 Concurrent Gastric Malignancy
In adults, symptomatic response to therapy with famotidine tablets does not preclude the presence of gastric malignancy. Consider evaluation for gastric malignancy in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with famotidine tablets.
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Famotidine tablets were studied in 7 U.S. and international placebo- and active-controlled trials in approximately 2500 patients [see Clinical Studies (14)] . A total of 1442 patients were treated with famotidine tablets, including 302 treated with 40 mg twice daily, 456 treated with 20 mg twice daily, 461 treated with 40 mg once daily, and 396 treated with 20 mg once daily. The population was 17-91 years old, fairly well distributed between gender and race, however, the predominant race treated was Caucasian.
The following adverse reactions occurred in greater than or equal to 1% of famotidine tablets-treated patients: headache, dizziness and constipation.
The following other adverse reactions were reported in less than 1% of patients in clinical trials:
Body as a Whole: fever, asthenia, fatigue
Cardiovascular: palpitations
Gastrointestinal: elevated liver enzymes, vomiting, nausea, abdominal discomfort, anorexia, dry mouth
Hematologic: thrombocytopenia
Hypersensitivity: orbital edema, rash, conjunctival injection, bronchospasm
Musculoskeletal: musculoskeletal pain, arthralgia
Nervous System/Psychiatric: seizure, hallucinations, depression, anxiety, decreased libido, insomnia, somnolence
Skin: pruritus, dry skin, flushing
Special Senses: tinnitus, taste disorder
Other: impotence
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of famotidine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: arrhythmia, AV block, prolonged QT interval
Gastrointestinal: cholestatic jaundice, hepatitis
Hematologic: agranulocytosis, pancytopenia, leukopenia
Hypersensitivity: anaphylaxis, angioedema, facial edema, urticaria
Musculoskeletal: rhabdomyolysis, muscle cramps
Nervous System/Psychiatric: confusion, agitation, paresthesia
Respiratory: interstitial pneumonia
Skin: toxic epidermal necrolysis/Stevens-Johnson syndrome
7 DRUG INTERACTIONS
7.1 Drugs Dependent on Gastric pH for Absorption
Famotidine can reduce the absorption of other drugs, due to its effect on reducing intragastric acidity, leading to loss of efficacy of the concomitant drug.
Concomitant administration of famotidine tablets with dasatinib, delavirdine mesylate, cefditoren, and fosamprenavir is not recommended.
See the prescribing information for other drugs dependent on gastric pH for absorption for administration instructions, including atazanavir, erlotinib, ketoconazole, itraconazole, ledipasvir/sofosbuvir, nilotinib, and rilpivirine.
7.2 Tizanidine (CYP1A2 Substrate)
Although not studied clinically, famotidine is considered a weak CYP1A2 inhibitor and may lead to substantial increases in blood concentrations of tizanidine, a CYP1A2 substrate. Avoid concomitant use with famotidine tablets. If concomitant use is necessary, monitor for hypotension, bradycardia or excessive drowsiness. Refer to the full prescribing information for tizanidine.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with H 2-receptor antagonists, including famotidine, in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, no adverse development effects were observed with oral administration of famotidine at doses up to approximately 243 and 122 times, respectively, the recommended human dose of 80 mg per day for the treatment of erosive esophagitis (see Data) .
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
Reproductive studies have been performed in rats and rabbits at oral doses of up to 2000 and 500 mg/kg/day, respectively, and in both species at intravenous doses of up to 200 mg/kg/day, and have revealed no significant evidence of impaired fertility or harm to the fetus due to famotidine tablets. While no direct fetotoxic effects have been observed, sporadic abortions occurring only in mothers displaying marked decreased food intake were seen in some rabbits at oral doses of 200 mg/kg/day (about 49 times the recommended human dose of 80 mg per day, based on body surface area) or higher. There are, however, no adequate or well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.2 Lactation
Risk Summary
There are limited data available on the presence of famotidine in human breast milk. There were no effects on the breastfed infant. There are no data on famotidine effects on milk production. Famotidine is present in the milk of lactating rats (see Data) .
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for famotidine and any potential adverse effects on the breastfed child from famotidine tablets or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of famotidine tablets have been established in pediatric patients for the treatment of peptic ulcer disease (i.e., duodenal ulcer, gastric ulcer) and GERD (i.e., symptomatic nonerosive GERD, erosive esophagitis as diagnosed by endoscopy). The use of famotidine tablets and the recommended dosage of famotidine tablets in these pediatric patients is supported by evidence from adequate and well-controlled studies of famotidine tablets in adults and published pharmacokinetic and pharmacodynamic data in pediatric patients [see Dosage and Administration (2.1), Clinical Pharmacology (12.2, 12.3)] . In pediatric patients, the safety and effectiveness for the treatment of pathological hypersecretory conditions and reduction of risk of duodenal ulcer recurrence have not been established.
Famotidine 20 and 40 mg tablets are not recommended for use in pediatric patients weighing less than 40 kg because these tablet strengths exceed the recommended dose for these patients [see Dosage and Administration (2.1)] . For pediatric patients weighing less than 40 kg, consider another famotidine formulation (e.g., oral suspension, lower dose tablet).
8.5 Geriatric Use
Of the 1442 famotidine tablets-treated patients in clinical studies, approximately 10% were 65 and older. In these studies, no overall differences in safety or effectiveness were observed between elderly and younger patients. In postmarketing experience, CNS adverse reactions have been reported in elderly patients with and without renal impairment receiving famotidine tablets [see Warnings and Precautions (5.1)] .
Famotidine is known to be substantially excreted by the kidney, and the risk of adverse reactions to famotidine tablets may be greater in elderly patients, particularly those with impaired renal function [see Use in Specific Populations (8.6)] .
In general, use the lowest effective dose of famotidine tablets for an elderly patient and monitor renal function [see Dosage and Administration (2.2)] .
8.6 Renal Impairment
CNS adverse reactions and prolonged QT intervals have been reported in patients with moderate and severe renal impairment [see Warnings and Precautions (5.1)] . The clearance of famotidine is reduced in adults with moderate and severe renal impairment compared to adults with normal renal function [see Clinical Pharmacology (12.3)] . No dosage adjustment is needed in patients with mild renal impairment (creatinine clearance greater than or equal to 60 mL/minute). Dosage reduction is recommended in adult and pediatric patients greater than or equal to 40 kg with moderate or severe renal impairment (creatinine clearance less than 60 mL/minute) [see Dosage and Administration (2.2)] .
10 OVERDOSAGE
The types of adverse reactions in overdosage of famotidine tablets are similar to the adverse reactions encountered with use of recommended dosages [see Adverse Reactions (6.1)] .
In the event of overdosage, treatment should be symptomatic and supportive. Unabsorbed material should be removed from the gastrointestinal tract, the patient should be monitored, and supportive therapy should be employed.
Due to low binding to plasma proteins, famotidine is eliminated by hemodialysis. There is limited experience on the usefulness of hemodialysis as a treatment for famotidine tablets overdosage.
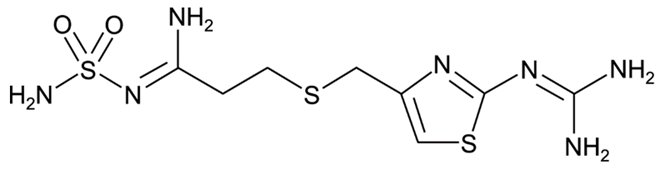
11 DESCRIPTION
The active ingredient in famotidine tablets, USP is a histamine-2 (H 2) receptor antagonist. Famotidine is propanimidamide, N'-(aminosulfonyl)-3-[[[2[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]-. The molecular formula of famotidine is C 8H 15N 7O 2S 3 and its molecular weight is 337.44. Its structural formula is:
Each famotidine tablet for oral administration contains either 20 mg or 40 mg of famotidine, USP and the following inactive ingredients: D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch (corn), sodium lauryl sulfate, sodium starch glycolate (potato), titanium dioxide and triacetin. In addition, the 20 mg tablet contains FD&C Blue No. 2 Aluminum Lake and the 40 mg tablet contains FD&C Blue No. 1 Aluminum Lake.
Famotidine, USP is a white to pale yellow crystalline compound that is freely soluble in glacial acetic acid, slightly soluble in methanol, very slightly soluble in water, and practically insoluble in ethanol.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Famotidine is a competitive inhibitor of histamine-2 (H 2) receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric secretion. Both the acid concentration and volume of gastric secretion are suppressed by famotidine, while changes in pepsin secretion are proportional to volume output.
12.2 Pharmacodynamics
Adults
Famotidine tablets inhibited both basal and nocturnal gastric secretion, as well as secretion stimulated by food and pentagastrin. After oral administration of famotidine tablets, the onset of the antisecretory effect occurred within one hour; the maximum effect was dose-dependent, occurring within one to three hours. Duration of inhibition of secretion by doses of 20 mg and 40 mg was 10 to 12 hours.
Single evening oral doses of 20 mg and 40 mg inhibited basal and nocturnal acid secretion in all subjects; mean nocturnal gastric acid secretion was inhibited by 86% and 94%, respectively, for a period of at least 10 hours. The same doses given in the morning suppressed food-stimulated acid secretion in all subjects. The mean suppression was 76% and 84%, respectively, 3 to 5 hours after administration, and 25% and 30%, respectively, 8 to 10 hours after administration. In some subjects who received the 20 mg dose, however, the antisecretory effect was dissipated within 6 to 8 hours. There was no cumulative effect with repeated doses. The nocturnal intragastric pH was raised by evening doses of 20 mg and 40 mg of famotidine tablets to mean values of 5.0 and 6.4, respectively. When famotidine tablets were given after breakfast, the basal daytime interdigestive pH at 3 and 8 hours after 20 mg or 40 mg of famotidine tablets was raised to about 5.
Famotidine tablets had little or no effect on fasting or postprandial serum gastrin levels. Gastric emptying and exocrine pancreatic function were not affected by famotidine tablets.
In clinical pharmacology studies, systemic effects of famotidine tablets in the CNS, cardiovascular, respiratory or endocrine systems were not noted. Also, no anti-androgenic effects were noted. Serum hormone levels, including prolactin, cortisol, thyroxine (T 4), and testosterone, were not altered after treatment with famotidine tablets.
Pediatric Patients
Pharmacodynamics of famotidine, assessed by gastric pH, were evaluated in 5 pediatric patients 2 to 13 years of age using the sigmoid E max model. These data suggest that the relationship between serum concentration of famotidine and gastric acid suppression is similar to that observed in adults (see Table 3).
|
|
|
|
EC 50(ng/mL)* |
|
|
Pediatric Patients |
26 ± 13 |
|
Adults | |
|
Healthy adult subjects |
26.5 ± 10.3 |
|
Adult patients with upper GI bleeding |
18.7 ± 10.8 |
In a study examining the effect of famotidine on gastric pH and duration of acid suppression in pediatric patients, four pediatric patients ages 11 to 15 years of age using the oral formulation at a dose of 0.5 mg/kg, maintained a gastric pH above 5 for 13.5 ± 1.8 hours.
12.3 Pharmacokinetics
Absorption
Famotidine is incompletely absorbed. The bioavailability of oral doses is 40 to 45%. Bioavailability may be slightly increased by food, or slightly decreased by antacids; however, these effects are of no clinical consequence.
Peak famotidine plasma levels occur in 1 to 3 hours. Plasma levels after multiple dosages are similar to those after single doses.
Elimination
Specific Populations
Pediatric Patients
Bioavailability studies of 8 pediatric patients (11 to 15 years of age) showed a mean oral bioavailability of 0.5 compared to adult values of 0.42 to 0.49. Oral doses of 0.5 mg per kg achieved AUCs of 580 ± 60 nghr/mL in pediatric patients 11 to 15 years of age, compared to 482 ± 181 nghr/mL in adults treated with 40 mg orally.
Patients with Renal Impairment
In adult patients with severe renal impairment (creatinine clearance less than 30 mL/minute), the systemic exposure (AUC) of famotidine increased at least 5-fold. In patients with moderate renal impairment (creatinine clearance between 30 to 60 mL/minute), the AUC of famotidine increased at least 2-fold [see Dosage and Administration (2.2), Use in Specific Population (8.6)] .
Drug Interaction Studies
Human Organic Anion Transporter (OAT) 1 and 3
In vitro studies indicate that famotidine is a substrate for OAT1 and OAT3. Following coadministration of probenecid (1500 mg), an inhibitor of OAT1 and OAT3, with a single oral 20 mg dose of famotidine in 8 healthy subjects, the serum AUC 0-10h of famotidine increased from 424 to 768 nghr/mL and the maximum serum concentration (C max) increased from 73 to 113 ng/mL. Renal clearance, urinary excretion rate and amount of famotidine excreted unchanged in urine were decreased. The clinical relevance of this interaction is unknown.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenic potential of famotidine was assessed in a 106-week oral carcinogenicity study in rats and a 92-week oral carcinogenicity study in mice. In the 106-week study in rats and the 92-week study in mice at oral doses of up to 2000 mg/kg/day (approximately 243 and 122 times, respectively, based on body surface area, the recommended human dose of 80 mg per day for the treatment of erosive esophagitis), there was no evidence of carcinogenic potential for famotidine.
Famotidine was negative in the microbial mutagen test (Ames test) using Salmonella typhimurium and Escherichia coli with or without rat liver enzyme activation at concentrations up to 10,000 mcg/plate. In in vivo studies in mice, with a micronucleus test and a chromosomal aberration test, no evidence of a mutagenic effect was observed.
In studies with rats given oral doses of up to 2000 mg/kg/day (approximately 243 times, based on body surface area, the recommended human dose of 80 mg per day) fertility and reproductive performance were not affected.
14 CLINICAL STUDIES
14.1 Active Duodenal Ulcer
In a U.S. multicenter, double-blind trial in adult outpatients with endoscopically confirmed duodenal ulcer (DU), orally administered famotidine tablets were compared to placebo. As shown in Table 4, 70% of patients treated with famotidine tablets 40 mg at bedtime were healed by Week 4. Most patients’ DU healed within 4 weeks.
Patients not healed by Week 4 were continued in the trial. By Week 8, 83% of patients treated with famotidine tablets had healed DU, compared to 45% of patients treated with placebo. The incidence of DU healing with famotidine tablets was greater than with placebo at each time point based on proportion of endoscopically confirmed healed DUs. Trials have not assessed the safety of famotidine tablets in uncomplicated active DU for periods of more than 8 weeks.
|
|
|||
|
Famotidine Tablets 40 mg at bedtime (N = 89) |
Famotidine Tablets 20 mg twice daily (N = 84) |
Placebo at bedtime (N = 97) |
|
|
Week 2 |
32% * |
38% * |
17% |
|
Week 4 |
70% * |
67% * |
31% |
In this study, time to relief of daytime and nocturnal pain was shorter for patients receiving famotidine tablets than for patients receiving placebo; patients receiving famotidine tablets also took less antacid than patients receiving placebo.
14.2 Active Gastric Ulcer
In both a U.S. and an international multicenter, double-blind trials in patients with endoscopically confirmed active gastric ulcer (GU), orally administered famotidine tablets 40 mg at bedtime were compared to placebo. Antacids were permitted during the trials, but consumption was not significantly different between the famotidine tablets and placebo groups.
As shown in Table 5, the incidence of GU healing confirmed by endoscopy (dropouts counted as unhealed) with famotidine tablets was greater than placebo at Weeks 6 and 8 in the U.S. trial, and at Weeks 4, 6 and 8 in the international trial. In these trials, most famotidine tablets-treated patients healed within 6 weeks. Trials have not assessed the safety of famotidine tablets in uncomplicated active GU for periods of more than 8 weeks.
|
|
||||
|
U.S. Study (N = 149) |
International Study (N = 294) |
|||
|
Famotidine Tablets 40 mg at bedtime (N = 74) |
Placebo at bedtime (N = 75) |
Famotidine Tablets 40 mg at bedtime (N = 149) |
Placebo at bedtime (N = 145) |
|
|
Week 4 |
45% |
39% |
47% * |
31% |
|
Week 6 |
66% * |
44% |
65% * |
46% |
|
Week 8 |
78% † |
64% |
80% * |
54% |
Time to complete relief of daytime and nighttime pain was statistically significantly shorter for patients receiving famotidine tablets than for patients receiving placebo; however, neither trial demonstrated a statistically significant difference in the proportion of patients whose pain was relieved by the end of the trial (Week 8).
14.3 Symptomatic Gastroesophageal Reflux Disease (GERD)
Orally administered famotidine tablets were compared to placebo in a U.S. trial that enrolled patients with symptoms of GERD and without endoscopic evidence of esophageal erosion or ulceration. As shown in Table 6, patients treated with famotidine tablets 20 mg twice daily had greater improvement in symptomatic GERD than patients treated with 40 mg at bedtime or placebo.
|
|
|||
|
Famotidine Tablets 20 mg twice daily (N = 154) |
Famotidine Tablets 40 mg at bedtime (N = 149) |
Placebo at bedtime (N = 73) |
|
|
Week 6 |
82% * |
69% |
62% |
14.4 Erosive Esophagitis Due to GERD
Healing of endoscopically verified erosion and symptomatic improvement were studied in a U.S. and an international double-blind trials. Healing was defined as complete resolution of all erosions visible with endoscopy. The U.S. trial comparing orally administered famotidine tablets 40 mg twice daily to placebo and orally administered famotidine tablets 20 mg twice daily showed a significantly greater percentage of healing of erosive esophagitis for famotidine tablets 40 mg twice daily at Weeks 6 and 12 (Table 7).
|
|
|||
|
Famotidine Tablets 40 mg twice daily (N = 127) |
Famotidine Tablets 20 mg twice daily (N = 125) |
Placebo twice daily (N = 66) |
|
|
Week 6 |
32% |
18% |
|
|
Week 12 |
54% * |
29% |
|
As compared to placebo, patients in the U.S. trial who received famotidine tablets had faster relief of daytime and nighttime heartburn, and a greater percentage of famotidine tablets-treated patients experienced complete relief of nighttime heartburn. These differences were statistically significant.
In the international trial, when orally administered famotidine tablets 40 mg twice daily were compared to orally administered ranitidine 150 mg twice daily, a statistically significantly greater percentage of healing of erosive esophagitis was observed with famotidine tablets 40 mg twice daily at Week 12 (Table 8). There was, however, no significant difference in symptom relief among treatment groups.
|
|
|||
|
Famotidine Tablets 40 mg twice daily (N = 175) |
Famotidine Tablets 20 mg twice daily (N = 93) |
Ranitidine 150 mg twice daily (N = 172) |
|
|
Week 6 |
48% |
52% |
42% |
|
Week 12 |
71% * |
68% |
60% |
14.5 Pathological Hypersecretory Conditions
In trials of patients with pathological hypersecretory conditions such as Zollinger-Ellison syndrome with or without multiple endocrine neoplasias, famotidine tablets significantly inhibited gastric acid secretion and controlled associated symptoms. Orally administered famotidine tablets dosages from 20 mg to 160 mg every 6 hours maintained basal acid secretion below 10 mEq/hour; initial dosages were titrated to the individual patient need and subsequent adjustments were necessary with time in some patients.
14.6 Risk Reduction of Duodenal Ulcer Recurrence
Two randomized, double-blind, multicenter trials in patients with endoscopically confirmed healed DUs demonstrated that patients receiving treatment with orally administered famotidine tablets 20 mg at bedtime had lower rates of DU recurrence, as compared with placebo.
- In the U.S. trial, DU recurrence within 12 months was 2.4 times greater in patients treated with placebo than in the patients treated with famotidine tablets. The 89 famotidine tablets-treated patients had a cumulative observed DU recurrence rate of 23%, compared to a 57% in the 89 patients receiving placebo (p < 0.01).
- In the international trial, the cumulative observed DU recurrence within 12 months in the 307 famotidine tablets-treated patients was 36%, compared to 76% in the 325 patients who received placebo (p < 0.01).
Controlled trials have not extended beyond one year.
16 HOW SUPPLIED/STORAGE AND HANDLING
Famotidine Tablets, USP are available containing 20 mg or 40 mg of famotidine, USP. They are available as follows:
The 20 mg tablets are yellow, film-coated, round, unscored tablets debossed with M over F1 on one side of the tablet and blank on the other side.
NDC: 0378-3020-01
bottles of 100 tablets
NDC: 0378-3020-05
bottles of 500 tablets
The 40 mg tablets are green, film-coated, round, unscored tablets debossed with M over F2 on one side of the tablet and blank on the other side.
NDC: 0378-3040-01
bottles of 100 tablets
Storage: Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
17 PATIENT COUNSELING INFORMATION
Central Nervous System (CNS) Adverse Reactions: Advise elderly patients and those with moderate and severe renal impairment of the risk of CNS adverse reactions, including confusion, delirium, hallucinations, disorientation, agitation, seizures, and lethargy [see Warnings and Precautions (5.1)] . Report symptoms immediately to a healthcare provider.
QT Prolongation: Advise patients with moderate and severe renal impairment of the risk of QT interval prolongation [see Use in Specific Populations (8.6)] . Report new cardiac symptoms, such as palpitations, fainting and dizziness or lightheadedness immediately to a healthcare provider.
Administration: Advise patients:
- Take famotidine tablets once daily before bedtime or twice daily in the morning and before bedtime, as recommended.
- Famotidine tablets may be taken with or without food.
- Famotidine tablets may be given with antacids.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Revised: 12/2018
FAMO:R12
DRUG: Famotidine
GENERIC: famotidine
DOSAGE: TABLET, FILM COATED
ADMINSTRATION: ORAL
NDC: 70518-1089-0
COLOR: yellow
SHAPE: ROUND
SCORE: No score
SIZE: 8 mm
IMPRINT: M;F1
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- FAMOTIDINE 20mg in 1
INACTIVE INGREDIENT(S):
- D&C YELLOW NO. 10
- SODIUM LAURYL SULFATE
- STARCH, CORN
- POVIDONE, UNSPECIFIED
- SODIUM STARCH GLYCOLATE TYPE A POTATO
- TITANIUM DIOXIDE
- TRIACETIN
- FD&C BLUE NO. 2
- FD&C YELLOW NO. 6
- HYPROMELLOSE, UNSPECIFIED
- LACTOSE MONOHYDRATE
- POLYDEXTROSE
- MAGNESIUM STEARATE
- MICROCRYSTALLINE CELLULOSE 112
- POLYETHYLENE GLYCOL, UNSPECIFIED
| FAMOTIDINE
famotidine tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |