SPF 32 Daily Suscreen by Ghost Democracy LLC / Inspec Solutions LLC / Inspec Solutions Ghost Democracy SPF 33
SPF 32 Daily Suscreen by
Drug Labeling and Warnings
SPF 32 Daily Suscreen by is a Otc medication manufactured, distributed, or labeled by Ghost Democracy LLC, Inspec Solutions LLC, Inspec Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPF 32 DAILY SUSCREEN GHOST DEMOCRACY- zinc oxide cream
Ghost Democracy LLC
----------
Ghost Democracy SPF 33
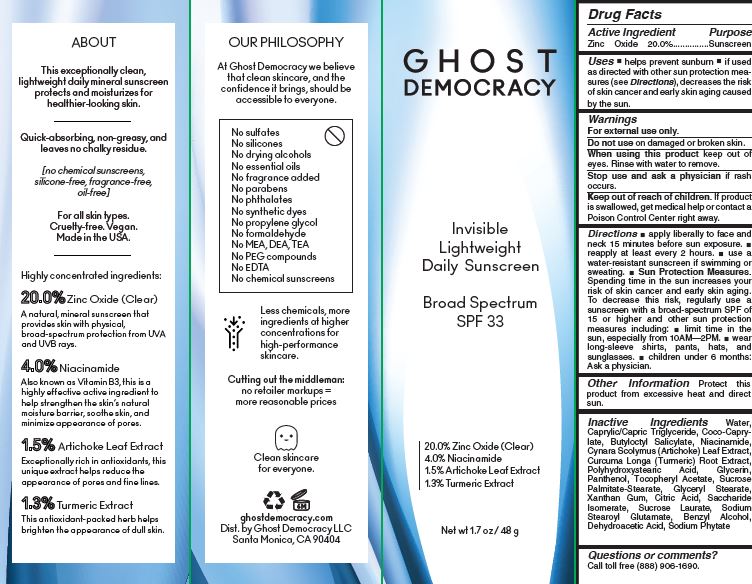
Uses
■ helps prevent sunburn ■ if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Do not useon damaged or broken skin.
When using this productkeep out of eyes. Rinse with water to remove.
Stop use and ask a physician ifrash occurs.
Keep out of reach of children.If product is swallowed, get medical help or contact a Poison Control Center right away
Directions
■ apply liberally to face and neck 15 minutes before sun exposure. ■ reapply at least every 2 hours. ■ use a water-resistant sunscreen if wimming or sweating. ■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including: ■ limit time in the
sun, especially from 10AM—2PM. ■ wear long-sleeve shirts, pants, hats, and sunglasses. ■ children under 6 months: Ask a physician.
Inactive Ingredients
Water,
Caprylic/Capric Triglyceride, Coco-Caprylate,
Butyloctyl Salicylate, Niacinamide,
Cynara Scolymus (Artichoke) Leaf Extract,
Curcuma Longa (Turmeric) Root Extract,
Polyhydroxystearic Acid, Glycerin,
Panthenol, Tocopheryl Acetate, Sucrose
Palmitate-Stearate, Glyceryl Stearate,
Xanthan Gum, Citric Acid, Saccharide
Isomerate, Sucrose Laurate, Sodium
Stearoyl Glutamate, Benzyl Alcohol,
Dehydroacetic Acid, Sodium Phytate
| SPF 32 DAILY SUSCREEN
GHOST DEMOCRACY
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Ghost Democracy LLC (117813286) |
| Registrant - Inspec Solutions LLC (081030372) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Inspec Solutions | 081030372 | manufacture(81412-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.