IMOXI FOR CATS- imidacloprid and moxidectin solution
IMOXI by
Drug Labeling and Warnings
IMOXI by is a Animal medication manufactured, distributed, or labeled by Vetoquinol USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
VETERINARY INDICATIONS
Once-a-month topical solution for cats for the prevention of heartworm disease, kills adult fleas, is indicated for the treatment of flea infestations, as well as the treatment and control of ear mite infestations and intestinal parasite infections in cats and kittens 9 weeks of age and older and that weigh at least 2 lbs.
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION:
IMOXI™ Topical Solution for Cats (10% imidacloprid + 1% moxidectin) is a colorless to yellow ready-to-use solution packaged in single-dose applicator tubes for topical treatment of cats. The formulation and dosage schedule are designed to provide a minimum of 4.5 mg/lb (10.0 mg/kg) imidacloprid and 0.45 mg/lb (1.0 mg/kg) moxidectin based on body weight.
Imidacloprid is a chloronicotinyl nitroguanidine insecticide. The chemical name of imidacloprid is 1-[(6-Chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine. Moxidectin is a semisynthetic macrocyclic lactone endectocide derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus. The chemical name of moxidectin is [6R, 23E, 25S(E)]-5-O-Demethyl-28-deoxy-25- (1,3-dimethyl-1-butenyl)-6,28-epoxy-23-(methoxyimino) milbemycin B.
-
INDICATIONS & USAGE
INDICATIONS:
IMOXI™ Topical Solution for Cats is indicated for the prevention of heartworm disease caused by Dirofilaria immitis. IMOXI™ Topical Solution for Cats kills adult fleas (Ctenocephalides felis) and is indicated for the treatment of flea infestations. IMOXI™ Topical Solution for Cats is also indicated for the treatment and control of ear mite (Otodectes cynotis) infestations and the following intestinal parasites:
Intestinal Parasite Intestinal Stage Adult Immature Adult Fourth Stage Larvae Hookworm Species Ancylostoma tubaeforme X X X Roundworm Species Toxocara cati X X -
WARNINGS
WARNINGS:
Do not use on sick, debilitated, or underweight cats (see ADVERSE REACTIONS). Do not use on cats less than 9 weeks of age or less than 2 lbs. body weight.
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children.
Children should not come in contact with the application site for 30 minutes after application.
Causes eye irritation. Harmful if swallowed. Do not get in eyes or on clothing. Avoid contact with skin. Exposure to the product has been reported to cause headache; dizziness; and redness, burning, tingling, or numbness of the skin. Wash hands thoroughly with soap and warm water after handling. If contact with eyes occurs, hold eyelids open and flush with copious amounts of water for 15 minutes. If eye irritation develops or persists, contact a physician. If swallowed, call poison control center or physician immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or physician. People with known hypersensitivity to benzyl alcohol, imidacloprid or moxidectin should administer the product with caution. In case of allergic reaction, contact a physician. If contact with skin or clothing occurs, take off contaminated clothing. Wash skin immediately with plenty of soap and water. Call a poison control center or physician for treatment advice.
The Safety Data Sheet (SDS) provides additional occupational safety information. To report suspected adverse drug events, for technical assistance or to obtain a copy of the SDS, contact Vetoquinol USA at 1-800-835-9496 or www.vetoquinolusa.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
PRECAUTIONS
PRECAUTIONS:
Do not dispense dose applicator tubes without complete safety and administration information.
Avoid oral ingestion. Cats may experience hypersalivation, tremors, vomiting and decreased appetite if IMOXI™ Topical Solution for Cats is inadvertently administered orally or through grooming/licking of the application site.
The safety of IMOXI™ Topical Solution for Cats has not been established in breeding, pregnant, or lactating cats.
The effectiveness of IMOXI™ Topical Solution for Cats against heartworm infections (D. immitis) after bathing has not been evaluated in cats.
Use of this product in geriatric patients with subclinical conditions has not been adequately studied. Several otherwise healthy, thin geriatric cats experienced prolonged lethargy and sleepiness after using imidacloprid and moxidectin topical solution. (See ADVERSE REACTIONS).
-
ADVERSE REACTIONS
ADVERSE REACTIONS:
Field Study: Following treatment with imidacloprid and moxidectin topical solution or an active control, cat owners reported the following post-treatment reactions:
OBSERVATION Imidacloprid and Moxidectin Topical Solution n=113 Active Control n=38 - * These three cats were from the same household and included one 13-yr-old cat in good health, one 15-yr-old FIV positive cat in good health, and one 15-yr-old, underweight cat in fair health. Lethargy was noted for 24 to 36 hrs after the first treatment only; one cat was unsteady at 48hrs. These cats were not on other medications.
Lethargy (protracted sleeping, poorly responsive) 3 cats*
(2.7%)None observed Behavioral changes (e.g., agitated, excessive grooming, hiding, pacing, spinning) 9 cats
(8.0%)1 cat (2.6%) Discomfort (e.g., scratching, rubbing, head-shaking) 5 cats
(4.4%)None observed Hypersalivation (within 1 hour after treatment) 3 cats
(2.7%)None observed Polydipsia 3 cats
(2.7%)None observed Coughing and gagging 1 cat (0.9%) None observed During another field study, a 16-year-old cat with renal disease slept in the same place without moving for two days following application. (See PRECAUTIONS).
Laboratory Effectiveness Studies: Imidacloprid and moxidectin topical solution was administered at the recommended dose to 215 cats in 20 effectiveness studies. One random-sourced cat exhibited signs consistent with either moxidectin toxicity or viral respiratory disease and died 26 hours after product application; necropsy findings were inconclusive as to the cause of death. A second cat that became ill 3 days after application of imidacloprid and moxidectin topical solution responded to treatment for respiratory infection and completed the study. A third cat became ill on day 3 and died with signs and lesions attributable to panleukopenia on day 7 after moxidectin application.
Post-Approval Experience: The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events in cats are listed in decreasing order of reporting frequency: hypersalivation, depression/lethargy, application site reactions (alopecia, pruritus, lesions, and erythema), decreased appetite, vomiting, hyperactivity, ataxia, trembling, and behavior disorder (hiding).
In some cases, death has been reported. In humans, ocular and dermal irritation, nausea, numbness or tingling of the mouth and lips, anaphylaxis, pruritus, vomiting, and tongue/taste abnormalities have been reported following exposure to imidacloprid and moxidectin topical solution.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the SDS, contact Vetoquinol USA at 1-800-835-9496 or www.vetoquinolusa.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
The recommended minimum dose is 4.5 mg/lb (10.0 mg/kg) imidacloprid and 0.45 mg/lb (1.0 mg/kg) moxidectin, once a month, by topical administration.
Do not apply to irritated skin.
1. Remove one dose applicator tube from the package. As specified in the following table, administer the entire contents of the IMOXI™ Topical Solution for Cats tube that correctly corresponds with the body weight of the cat.
Cat (lbs.) Volume (mL) Imidacloprid (mg) Moxidectin (mg) - * Cats over 18 lbs. should be treated with the appropriate combination of IMOXI™ Topical Solution for Cats tubes.
2-5 0.23 23 2.3 5.1-9 0.4 40 4 9.1-18* 0.8 80 8 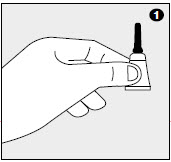
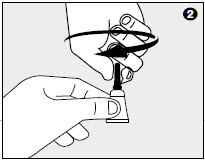
2. While holding the TwistNGo™ tube in an upright position, twist dispensing tip clockwise about ½ turn to break the tube's seal. Remove the cap from the tube.
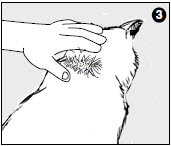
3. Part the hair on the back of the cat's neck at the base of the head, until the skin is visible.
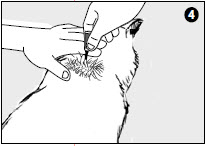
4. Place the tip of the tube on the skin and apply the entire contents directly on the exposed skin. Lift the tube away from the skin before releasing pressure on the tube.
Do not get this product in the cat's mouth or eyes or allow the cat to lick the application site for 30 minutes. Treatment at the base of the head will minimize the opportunity for ingestion by grooming. In households with multiple pets, keep animals separated to prevent licking of the application site.
Stiff, matted hair or a damp, oily appearance of the hair may be observed at the application site on some cats. This is temporary and does not affect the safety and effectiveness of the product.
Heartworm Prevention: For prevention of heartworm disease, IMOXI™ Topical Solution for Cats should be administered at one-month intervals. IMOXI™ Topical Solution for Cats may be administered year-around or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer IMOXI™ Topical Solution for Cats immediately and resume the monthly dosing schedule. When replacing another heartworm preventative product in a heartworm prevention program, the first treatment with IMOXI™ Topical Solution for Cats should be given within one month of the last dose of the former medication. At the discretion of the veterinarian, cats older than 6 months of age may be tested to determine the presence of existing heartworm infection before treatment with IMOXI™ Topical Solution for Cats. (See ADVERSE REACTIONS – Post-Approval Experience).
Flea Treatment: For the treatment of flea infestations, IMOXI™ Topical Solution for Cats should be administered at one-month intervals. If the cat is already infested with fleas when the first dose of IMOXI™ Topical Solution for Cats is administered, adult fleas on the cat will be killed. However, re-infestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated. Cats treated with imidacloprid, including those with pre-existing flea allergy dermatitis, have shown clinical improvement as a direct result of elimination of fleas from the cat.
Ear Mite Treatment: For the treatment of ear mites (Otodectes cynotis), IMOXI™ Topical Solution for Cats should be administered once as a single topical dose. Monthly use of IMOXI™ Topical Solution for Cats will control any subsequent ear mite infestations.
Intestinal Nematode Treatment: For the treatment and control of intestinal hookworm infections caused by Ancylostoma tubaeforme (adults, immature adults and fourth stage larvae) and roundworm infections caused by Toxocara cati (adults and fourth stage larvae), IMOXI™ Topical Solution for Cats should be administered once as a single topical dose.
-
SPL UNCLASSIFIED SECTION
ANIMAL SAFETY:
Studies in Kittens: Imidacloprid and moxidectin topical solution was topically applied at 0, 1, 3, and 5× the maximum dose to 48 healthy 9-week-old kittens on days 0, 28, and 56. Lethargy was observed in 1 kitten from the 3× group and 1 from the 5× group on the day after initial treatment; the kitten from the 3× group was also disoriented and ataxic. One kitten from the 3× group had a slow pupillary light response two days after treatment and one had tremors the day after treatment. Hypersalivation was seen in one kitten from the 5× group approximately six hours post-treatment. One kitten from the 3× group was scratching at the treatment site 2 days after treatment. Slight cough was noted in 7 different kittens (2-0×, 2-1×, and 3-5×) during the 13-day period following the first treatment. Histopathology showed granulomatous inflammation at the treatment site in three 1× kittens. Causal relationship to the drug could not be determined. Pulmonary inflammation (1-5×) and lymphoid hyperplasia (2-1×, 4-3×) were seen in treated kittens. In a second study, imidacloprid and moxidectin topical solution was topically applied at 0, 1.7, 5.2 and 8.7× the maximum dose to 48 healthy 9-week-old kittens every two weeks for 6 doses. One kitten in the 8.7× group apparently ingested an unknown amount of the drug and developed the following clinical signs prior to euthanasia: mydriasis, salivation, depression, vomiting, unsteadiness, rapid to slow to difficult breathing, poor pupillary response, generalized tremors, inability to move, and nystagmus. Two kittens in the 5.2× group developed mydriasis, salivation, depression, squinting, and poor appetite. A kitten in the 1.7× group developed mydriasis.
Dose Tolerance Study: Eight healthy juvenile cats were topically dosed with a single application of imidacloprid and moxidectin topical solution at 10 times the recommended dose volume. Mild, transient hypersalivation occurred in two of the cats.
Oral Study in Cats: The oral safety of imidacloprid and moxidectin topical solution was tested in case of accidental oral ingestion. The maximum topical dose was orally administered to twelve healthy 9-week-old kittens. Hypersalivation (8 of 12 kittens) and vomiting (12 of 12 kittens) were observed immediately post-treatment. Tremors developed in one kitten within 1 hour, resolving without treatment within the next hour. All 12 kittens were either anorexic or had decreased appetite for at least 1 day following treatment. In 3 kittens, the anorexia or decreased appetite continued into the second week following treatment. There was a post-treatment loss of body weight in treated kittens compared to control kittens. In a pilot safety study using kittens younger in age and lighter in weight than allowed by product labeling, an 8-week-old kitten weighing 0.6 kg orally received 2× of the label topical dose (0.46 mL/kg). Immediately after dosing, it vomited, had labored breathing and slight tremors. Within 4 hours, it was normal, but was found dead on day 6. Necropsy could not determine the cause of death.
Study in Heartworm Positive Cats: Young adult cats were inoculated subcutaneously with third-stage D. immitis larvae. At 243-245 days post-infection, immunoserology and echocardiography were performed to identify cats with adult heartworm burdens similar to naturally-acquired infections. Two groups were treated topically with either imidacloprid and moxidectin topical solution at the label dose or placebo, once every 28 days, for three consecutive treatments. A third group was treated topically, once, with imidacloprid and moxidectin topical solution at 5× the label dose. Sporadic vomiting and labored breathing related to heartworm burden were observed in the treatment and control groups. There was no difference between treatment groups in the numbers of adult D. immitis recovered at study conclusion. No adverse reactions were associated with the topical application of imidacloprid and moxidectin topical solution to experimentally heartworm-infected cats.
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 0.23 mL Tube Blister Pack Carton
vetoquinol
NDC: 17030-023-03
CAUTION: Federal (U.S.A.)
Law restricts this drug
to use by or on the order
of a licensed veterinarian.Each tube contains 23 mg
of imidacloprid and 2.3 mg
of moxidectin.Children should not
come in contact with the
application site for 30
minutes after application.Keep this and all drugs
out of reach of children.3 PACK
THREE 0.23 mL TubesIMOXI™ Topical Solution for Cats
(imidacloprid + moxidectin)Once-A-Month Topical Solution
For Cats and Kittens
9 Weeks of Age and Older
and 2 to 5 lbs.Approved by FDA under ANADA # 200-638
2 - 5 lbs.
- Prevention of
heartworm
disease - Treatment and
control of ear
mite infestations - Kills adult fleas
and is indicated
for the treatment
of flea infestations - Treatment and
control of
hookworms - Treatment and
control of
roundworms

- Prevention of
-
PRINCIPAL DISPLAY PANEL - 0.4 mL Tube Blister Pack Carton
vetoquinol
NDC: 17030-044-06
CAUTION: Federal (U.S.A.) Law
restricts this drug to use by or
on the order of a licensed
veterinarian.Each tube contains 40 mg
of imidacloprid and 4 mg
of moxidectin.Children should not come in
contact with the application
site for 30 minutes after
application.Keep this and all drugs out of
reach of children.6 PACK
SIX 0.4 mL TubesIMOXI™ TOPICAL SOLUTION FOR CATS
(imidacloprid + moxidectin)Once-A-Month Topical Solution
For Cats and Kittens
9 Weeks of Age and Older
and 5.1 to 9 lbs.Approved by FDA under ANADA # 200-638
5.1 - 9 lbs.
- Prevention of
heartworm
disease - Treatment and
control of ear
mite infestations - Kills adult fleas
and is indicated
for the treatment
of flea infestations - Treatment and
control of
hookworms - Treatment and
control of
roundworms

- Prevention of
-
PRINCIPAL DISPLAY PANEL - 0.8 mL Tube Blister Pack Carton
vetoquinol
NDC: 17030-080-06
CAUTION: Federal (U.S.A.) Law
restricts this drug to use by or
on the order of a licensed
veterinarian.Each tube contains 80 mg
of imidacloprid and 8 mg of
moxidectin.Children should not come in
contact with the application
site for 30 minutes after
application.Keep this and all drugs out of
reach of children.6 PACK
SIX 0.8 mL TubesIMOXI™ TOPICAL SOLUTION FOR CATS
(imidacloprid + moxidectin)Once-A-Month Topical Solution
For Cats and Kittens
9 Weeks of Age and Older
and 9.1 to 18 lbs.Approved by FDA under ANADA # 200-638
9.1 - 18 lbs.
- Prevention of
heartworm
disease - Treatment and
control of ear
mite infestations - Kills adult fleas
and is indicated
for the treatment
of flea infestations - Treatment and
control of
hookworms - Treatment and
control of
roundworms

- Prevention of
-
INGREDIENTS AND APPEARANCE
IMOXI FOR CATS
imidacloprid and moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17030-023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIDACLOPRID (UNII: 3BN7M937V8) (IMIDACLOPRID - UNII:3BN7M937V8) IMIDACLOPRID 100 mg in 1 mL MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17030-023-03 1 in 1 CARTON 1 NDC: 17030-023-01 3 in 1 BLISTER PACK 1 0.23 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200638 12/08/2020 IMOXI FOR CATS
imidacloprid and moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17030-044 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIDACLOPRID (UNII: 3BN7M937V8) (IMIDACLOPRID - UNII:3BN7M937V8) IMIDACLOPRID 100 mg in 1 mL MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17030-044-06 1 in 1 CARTON 1 NDC: 17030-044-01 6 in 1 BLISTER PACK 1 0.40 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200638 12/08/2020 IMOXI FOR CATS
imidacloprid and moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17030-080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIDACLOPRID (UNII: 3BN7M937V8) (IMIDACLOPRID - UNII:3BN7M937V8) IMIDACLOPRID 100 mg in 1 mL MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17030-080-06 1 in 1 CARTON 1 NDC: 17030-080-01 6 in 1 BLISTER PACK 1 0.80 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200638 12/08/2020 Labeler - Vetoquinol USA, Inc. (106824209)
Trademark Results [IMOXI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMOXI 88481176 not registered Live/Pending |
Piedmont Animal Health, LLC 2019-06-20 |
 IMOXI 86943829 not registered Live/Pending |
PIEDMONT ANIMAL HEALTH, LLC 2016-03-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.