IMMITICIDE- melarsomine dihydrochloride and water kit
IMMITICIDE by
Drug Labeling and Warnings
IMMITICIDE by is a Animal medication manufactured, distributed, or labeled by Merial, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
CAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING
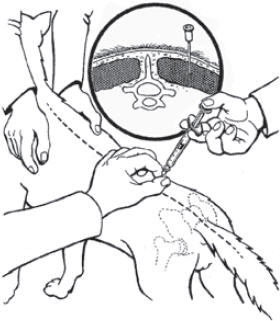
IMMITICIDE® should be administered by deep intramuscular injection in the lumbar (epaxial) muscles (L3 - L5) ONLY.
DO NOT USE IN ANY OTHER MUSCLE GROUP. DO NOT USE INTRAVENOUSLY.
Care should be taken to avoid superficial injection or leakage. (See SAFETY). - ACTIVE INGREDIENT
-
PHARMACOLOGY
Melarsomine dihydrochloride is an organic arsenical chemotherapeutic agent. Melarsomine has a molecular weight of 501.34 and is chemically designated as 4 – [(4, 6-diamino-1, 3, 5-triazon-2-yl) amino] phenyl-dithioarsenite of di (2-aminoethyl), dihydrochloride. It is freely soluble in water. When injected intramuscularly, it is rapidly absorbed. The exact mode of action on D. immitis is unknown.
-
INDICATIONS
IMMITICIDE Sterile Power is indicated for the treatment of stabilized Class 11, 22, and 33 heartworm disease caused by immature (4 month-old, stage L5) to mature adult infections of Dirofilaria immitis in dogs.
Heartworm Disease Classification: The following parameters were used to classify the dogs in the clinical field trials for IMMITICIDE. Other parameters may be considered. As a general rule, conservative treatment should be employed since heartworm disease is serious and potentially fatal. If there is evidence of a high worm burden, patients should be categorized as Class 3.
- 1 Class 1: Patients in this category are characterized as having asymptomatic to mild heartworm disease. No radiographic signs or signs of anemia are evident. Patients with mild disease may have subjective signs such as a general loss of condition, fatigue on exercise, or occasional cough; however, no objective radiographic or other abnormal laboratory parameters will be present.
- 2 Class 2: Patients in this category are characterized as having moderate heartworm disease. Radiographic signs or signs of anemia [Packed Cell Volume (PCV) less than 30% but greater than 20%, or other hematologic parameters below normal] are evident. Mild proteinuria (2+) may be present. Radiographic signs may include right ventricular enlargement, slight pulmonary artery enlargement, or circumscribed perivascular densities plus mixed alveolar/interstitial lesions. Patients may be free of subjective clinical signs or may have a general loss of condition, fatigue on exercise, or occasional cough. If necessary, patients should be stabilized prior to treatment.
- 3 Class 3: Patients in this category are characterized as having severe heartworm disease. These patients have a guarded prognosis. Subjective signs of disease may include cardiac cachexia (wasting), constant fatigue, persistent cough, dyspnea, or other signs associated with right heart failure such as ascites and/or jugular pulse. Radiographic signs may include right ventricular enlargement or right ventricular plus right atrial enlargement, severe pulmonary artery enlargement, circumscribed to chronic mixed patterns and diffuse patterns of pulmonary densities or radiographic signs of thromboembolism. Signs of significant anemia (PCV <20% or other hematologic abnormalities) may be present. Proteinuria (>2+) may be present. Patients may have only moderate clinical signs and significant laboratory or radiographic alterations or they may have significant clinical signs with only moderate laboratory and radiographic signs and be categorized as Class 3. Patients in Class 3 should be stabilized prior to treatment and then administered the alternate dosing regime (See PRECAUTIONS and DOSAGE AND ADMINISTRATION).
- CONTRAINDICATIONS
-
WARNINGS
(See boxed Warning). For use in dogs only. Safety for use in breeding animals and lactating or pregnant bitches has not been determined.
-
HUMAN WARNINGS
Keep this and all medications out of the reach of children. Avoid human exposure. Wash hands thoroughly after use or wear gloves. Potentially irritating to eyes. Rinse eyes with copious amounts of water if exposed. Consult a physician in cases of accidental exposure by any route (dermal, oral, or by injection).
The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information. To report adverse effects, obtain an MSDS or for assistance, contact Merial at 1-888-637-4251, option 3.
-
PRECAUTIONS
General: All dogs, with heartworm disease are at risk for post-treatment pulmonary thromboembolism (death of worms which may result in fever, weakness, and coughing), though dogs with severe pulmonary arterial disease have an increased risk and may exhibit more severe signs (dyspnea, hemoptysis, right heart failure and possibly death). Dogs should be restricted from light to heavy exercise post-treatment depending on the severity of their heartworm disease.
Studies in healthy (heartworm negative) dogs indicate that adverse reactions may occur after the second injection in the series even if no problems were encountered with the first injection. All patients should be closely monitored during treatment and for up to 24 hours after the last injection.
Special Considerations for Class 3 dogs: Following stabilization, severely ill (Class 3) dogs should be treated according to the alternate dosing regime in an attempt to decrease post-treatment mortality associated with thromboembolism. (See DOSAGE AND ADMINISTRATION). Post-treatment mortality due to thromboembolism and/or progression of the underlying disease may occur in 10 to 20% of the Class 3 patients treated with IMMITICIDE (see Mortality). Hospitalization post-treatment and strict exercise restriction are recommended. Other supportive therapies should be considered on a case-by-case basis.
If the alternate dosing regime is used, expect increased injection site reactions on the side receiving the second injection since the skeletal muscles at the first injection site may not have fully recovered (healed). If persistent swelling is present at 1 month, the second injections may be delayed for several weeks up to 1 month.
-
SAFETY
IMMITICIDE has a low margin of safety. A single dose of 7.5 mg/kg (3× the recommended dose) can result in pulmonary inflammation, edema, and death. Daily administration of 2× and 3× the recommended dose for 6 days caused no renal injury; however, daily administration of these doses for 14 days caused renal damage in healthy dogs. Adverse reactions, primarily at the injection sites, were seen at the recommended dose in clinical trials (see ADVERSE REACTIONS).
Studies in Healthy (Heartworm Negative) Dogs: The safety of IMMITICIDE was studied in 24 healthy beagle dogs. Drug was administered at 0, 2.5, 5.0 and 7.5 mg/kg for 6 consecutive days (0, 1, 2 and 3 times the recommended dosage). Clinical observations included tremors, lethargy, unsteadiness/ataxia, restlessness, panting, shallow and labored respiration, and/or rales. These signs were seen in all groups treated with IMMITICIDE with frequency and intensity increasing with increasing dosage. Death or euthanasia in a moribund state occurred in 3/6 dogs in the 7.5 mg/kg (3×) group. The signs exhibited by these dogs, in addition to the signs described above, included collapse, severe salivation, vomiting, respiratory distress, cyanosis, stupor, and death within 4 hours of the first dose in two dogs and within 20 hours of the second dose in one dog.
Body weights, water consumption, hematology and urine parameters were comparable to controls. Decreased food consumption occurred sporadically in the two high dose groups. Elevations, up to 25-fold, in creatinine kinase (CK) and elevations, up to 7-fold, in aspartate aminotransferase (AST) were observed and related grossly and histologically to muscle damage at the injection sites. Up to 2-fold elevations in alanine aminotransferase (ALT) were also noted. Gross and microscopic pathology revealed no organ-related toxicity other than edema and acute inflammation in the lungs and pleural effusion in the 3 dogs that died at the 7.5 mg/kg dose. Injection site irritation was observed in the skeletal muscles at all dose levels. At 5.0 mg/kg an injection site abscess was observed in one dog.
A separate study was conducted to examine the intensity and duration of injection site reactions. The dogs were dosed at 2.5 and 5.0 mg/kg (1× and 2× the recommended dose) twice 24 hours apart. This treatment series was repeated 4 months later. One group received the second treatment series after 1 month to mimic the alternate dosing regime. Swelling, which occurred within 7 days of injection and persisted from 1 to 72 days (average 30 days), was the most common clinical observation. A small, firm nodule in the lumbar region of one dog in the 1× group appeared during the first month of the study and persisted for 41 days. Pain at or following injection was not observed in this study. Elevations of the same magnitude as in the previous study and again related to muscle damage were observed in CK and AST within 8 hours of injection. The values approached pretest levels by 72 hours and were within the normal range established by control animals by 1 month post-injection.
Gross and microscopic evidence of injection site irritation (cellular infiltrate, fibrosis, necrosis, and hemorrhage) was still evident in the muscles 1 month post-injection in dogs at both dose levels. By 3 months post-injection, resolution (healing) was evident microscopically in the skeletal muscles at the 2.5 mg/kg dose level. One dog treated at the 2× dose had extension of treatment-related injection site inflammation into deeper tissues (i.e., abdominal cavity) as evidenced by an adhesion between the spleen and mesentery.
-
ADVERSE REACTIONS (Side Effects)
Injection Sites: At the recommended dosage in clinical field trials, significant irritation was observed at the intramuscular injection sites, accompanied by pain, swelling, tenderness, and reluctance to move. Approximately 30% of treated dogs experienced some kind of reaction at the injection site(s). Though injection site reactions were generally mild to moderate in severity and recovery occurred in 1 week to 1 month, severe reactions did occur (<1.0%), so care should be taken to avoid superficial or subcutaneous injection and leakage. Firm nodules can persist indefinitely.
Other Reactions: Coughing/gagging, depression/lethargy, anorexia/inappetence, fever, lung congestion, and vomiting were the most common reactions observed in dogs treated with IMMITICIDE. Hypersalivation and panting occurred rarely in clinical trials (1.9% and 1.6%, respectively); however, these signs may occur within 30 minutes of injection and may be severe. One dog vomited after each injection of IMMITICIDE, despite pre-treatment with anti-emetics. All adverse reactions resolved with time or treatment with the exception of a limited number of injection site reactions (persistent nodules, (see Table: Average Onset Time and Duration (with Ranges) of the Most Common Reactions in Clinical Trials) and a low number of post-treatment deaths. (See Mortality).
Prevalence of Clinical Observations/Adverse Reactions Reported in Clinical Field Trials: The following table enumerates adverse events that occurred in 1.5% or more of dogs with Class 1, 2, and 3 heartworm disease treated with IMMITICIDE in clinical field trials. Comparison is made with the same adverse events reported in dogs treated with placebo. Some of the following clinical observations/adverse reactions seen in dogs treated with IMMITICIDE may be directly attributable to the drug or they may be secondary to worm death and/or the underlying heartworm disease process.
Prevalence of Clinical Observation/Adverse Reactions Reported in Clinical Field Trials Clinical Observation/Adverse Reaction IMMITICIDE
% of dogs
n=311PLACEBO
% of dogs
n=63Injection Site Reactions
32.8
3.2
Coughing/Gagging
22.2
14.3
Depression/Lethargy
15.4
4.8
Anorexia/Inappetence
13.2
3.2
Pyrexia (fever)
7.4
0.0
Lung Congestion/Sounds
5.5
1.6
Emesis
5.1
1.6
Diarrhea
2.6
0.0
Dyspnea
2.6
1.6
Hypersalivation
1.9
0.0
Panting
1.6
0.0
Hemoptysis
1.6
0.0
Clinical observations/adverse reactions occurring in less than 1.5% of the dogs treated with IMMITICIDE include: abdominal hemorrhage, abdominal pain, bloody stool/diarrhea, colitis, gingivitis, pancreatitis, anemia, DIC, hemoglobinemia, icterus (mucous membranes), discoloured urine, hematuria, inappropriate urination, low specific gravity, polyuria, pyuria, bronchitis, miscellaneous respiratory problem, pneumonia, tachypnea, tracheobronchitis, wheezing, alopecia, hair color and coat character change, miscellaneous skin problem, ataxia, disorientation, fatigue/tires easily, miscellaneous eye problem, weight loss, convulsion/seizure, leukocytosis, polydipsia, and restlessness.
Onset and Duration of Clinical Observations/Adverse Reactions: The following table is provided to show the average onset time post-treatment for the most common reactions and the average duration of each event, as calculated from the 311 dogs treated with IMMITICIDE in the clinical field trials.
Average Onset Time and Duration (with Ranges) of the Most Common Reactions in Clinical Trials Clinical Observation/Adverse Reaction Avg. Onset Time in Days
(range)*Avg. Duration in Days
(range)*- * A zero indicates that the reaction first occurred on the day of treatment.
Injection Site
Swelling/Edema/Seroma
6 (0*-77)
18 (<1-210)
Pain/Discomfort/Irritation Inflammation/Heat
1 (0-6)
3 (<1-30)
Generalized/Local Myalgia with Tenderness and Stiffness
3 (1-8)
9 (<1-30)
Persistent (lumps, knots, nodules, masses)
22 (0-99)
47 (1-152)
Abscess (sterile and septic)
24 (10-42)
21 (5-36)
Coughing/Gagging
10 (0-103)
13 (<1-134)
Depression/Lethargy
5 (0-46)
6 (<1-48)
Anorexia/Inappetence
5 (0-63)
5 (<1-30)
Mortality: Death is a possible sequelae of heartworm disease in dogs with or without treatment, especially in the Class 3 dogs. The following table shows the percentage of dogs that died in clinical trials with IMMITICIDE® (melarsomine dihydrochloride) and the causes of death, if known.
Mortality in Dogs with Class 1, 2, and 3 Heartworm Disease Treated with IMMITICIDE in Clinical Field Trials Class 1, 2
% of dogs
n=267Class 3
% of dogs
n=44Total Deaths:
5.2
18.2
Cause:
Trauma
2.3
2.3
Thromboembolism
0.0
4.6
Euthanasia
(unrelated to treatment or underlying disease)1.1
0.0
Euthanasia
(related to treatment or underlying disease)0.0
2.3
Underlying Disease
0.8
2.3
Undetermined
1.1
6.8
In one small (n=15), uncontrolled field study in severely ill (Class 3) dogs, 5 dogs died following treatment. Pulmonary thromboembolism was the cause of one death. The remaining dogs were not necropsied. All 5 dogs were in right heart failure at the time of treatment. Clinical signs seen in this study which were not seen in the larger studies include atrial fibrillation, collapse, hypothermia, and weakness.
Post Approval Experience: In addition to the aforementioned adverse reactions reported in pre-approval clinical studies, there have also been rare reports of paresis and paralysis in dogs following administration of IMMITICIDE. To report a suspected adverse reaction, contact Merial at 1-888-637-4251, option-3.
Overdosage: Three dogs were inadvertently overdosed with IMMITICIDE in the clinical field trials when the dose was calculated on a mg/lb basis rather than a mg/kg basis (2× overdosage). Within 30 minutes of injection, one dog showed excessive salivation, panting, restlessness, and fever with all signs resolving within 4 hours. Vomiting and diarrhea were seen in the second dog within 24 hours of injection. The dog vomited once and the diarrhea resolved within 24 hours. The third dog showed no systemic reaction to the overdosage. Clinical observations in healthy beagle dogs after receiving up to 3× the recommended dose included tremors, lethargy, unsteadiness/ataxia, restlessness, panting, shallow and labored respiration, rales, severe salivation, and vomiting which progressed to respiratory distress, collapse, cyanosis, stupor, and death. (See SAFETY).
BAL in Oil Ampules (Dimercaprol Injection, USP) [Akorn, San Clemente, California, at 1-800-223-9851] is reported in the literature to be an antidote for arsenic toxicity and was shown in one study to reduce the signs of toxicity associated with overdosage of IMMITICIDE. The efficacy of IMMITICIDE may be reduced with co-administration of BAL.
-
SPL UNCLASSIFIED SECTION
EFFICACY
Results of the laboratory and clinical field trials demonstrate that treatment with IMMITICIDE results in reduction and/or clearance of D. immitis infection in dogs with Class 1, 2, and 3 heartworm disease. Evaluations for efficacy were determined by post-mortem worm counts in the laboratory studies and detection of antigen in the blood and subjective clinical assessments in the clinical trials. Physical exams, assessments of clinical variables, class of heartworm disease, radiographic examinations, as well as complete blood counts, serum chemistry profiles, and urinalysis were evaluated in the field trials.
Laboratory Studies: In placebo-controlled laboratory studies, IMMITICIDE, administered at 2.5 mg/kg twice, 24 hours apart, was 90.7% effective against transplanted adult heartworms and 90.8% effective against induced infections of 4 months old (L5) immature heartworms. To evaluate the effectiveness of the alternate dosing regimen, dogs with transplanted heartworms were treated with either 2.5 mg/kg once or 2.5 mg/kg once followed 1 month later with 2.5 mg/kg administered twice 24 hours apart. A single injection of IMMITICIDE at 2.5 mg/kg reduced male worms 87.7% and female worms 16.9% (total 51.7%). When the full regime was used 100% of male worms and 98% of female worms were killed (total 99%). Dogs with natural D. immitis infections were treated with IMMITICIDE at 2.5 mg/kg twice, 24 hours apart. This dose was repeated 4 months later. Antigen tests performed at month 4 showed a 90% conversion from antigen positive to negative status. Worm counts at month 9 showed a 98.7% reduction in worm numbers as compared to placebo controls.
Clinical Field Studies: In two well-controlled field studies, 169 client-owned dogs, 1 to 12 years old and weighing 3.0 to 59.0 kg, with Class 1 or stabilized Class 2 heartworm disease were treated with the recommended dose of IMMITICIDE. In-office blood antigen tests were used pre-treatment to diagnose the D. immitis infection and 4 months after drug administration to assess treatment response. At month 4, 76.2 to 81% of the dogs had converted from antigen positive to antigen negative status. The conversion rate ranged from 89.7 to 98.2% after two treatment series. In an open-label study in 102 dogs, 1 to 18 years old and weighing 4.4 to 40.8 kg, with Class 1 or stabilized Class 2 heartworm disease, the conversion rate was 84% 4 months after one series of treatments. When a second series was given at month 4, the conversion rate was 94%.
An open-label clinical field study was conducted in 44 dogs, 1.5 to 14 years old and weighing 3.2 to 50.0 kg, with stabilized, Class 3 heartworm disease. Dogs received the alternate dosing regime (2.5 mg/kg once followed 1 month later by 2.5 mg/kg twice 24 hours apart). The conversion rate was 89.2% 4 months after the final treatment. In a small, uncontrolled field trial (n=10) in Class 3 dogs the conversion rate was 100% 4 months after treatment.
-
DOSAGE AND ADMINISTRATION
IMMITICIDE should be administered ONLY by deep intramuscular injection in the epaxial (lumbar) muscles in the third through fifth lumbar region (see graphic). DO NOT ADMINISTER AT ANY OTHER SITE. Avoid superficial injection or leakage. Use a 23 gauge 1 inch needle for dogs equal to or less than 10 kg (22 lb) in weight. Use a 22 gauge 1 ½ inch needle for dogs greater than 10 kg (22 lb). Use alternating sides with each administration. If repeated administrations are warranted avoid injecting at the same lumbar location. Record the location of the first injection(s) in the patient's medical record for future reference.
Disease Classification: It is vital to classify the severity of heartworm disease to apply the appropriate dosage regime for IMMITICIDE. (See INDICATIONS).
Class 1 and 2:
If necessary, dogs should be stabilized prior to treatment. IMMITICIDE should be administered intramuscularly in the lumbar (L3 - L5) muscles at a dose of 2.5 mg/kg twice, 24 hours apart. (See Dosing Table). Four months following treatment, a second treatment series (2.5 mg/kg twice, 24 hours apart) can be elected taking into consideration the response to the first IMMITICIDE treatment and the condition, age, and use of the dog. Worms that were too young to be killed by the first treatment series, i.e., <4 months, may be killed by a second treatment series.
Class 3:
Alternate Dosing Regime: Dogs with severe (Class 3) heartworm disease should be stabilized prior to treatment and then dosed intramuscularly in the lumbar (L3 - L5) muscles with a single injection of 2.5 mg/kg then approximately 1 month later with 2.5 mg/kg administered twice 24 hours apart. (See Dosing Table).
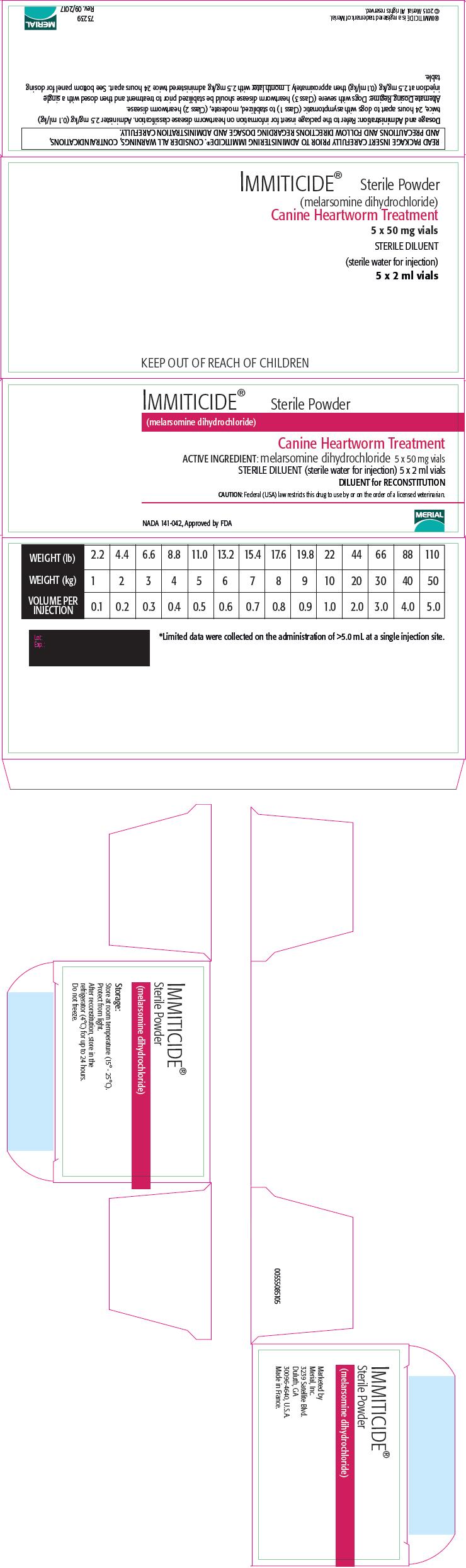
Dosing Table: Care must be taken to administer the proper dose. Accurately weigh the dog and calculate the volume to be injected based on the dose of 2.5 mg/kg (1.1 mg/lb). This is equivalent to 0.1 mL/kg (0.045 mL/lb). The following table should be used as a guide to ensure that the proper volume has been calculated.
Preparation: IMMITICIDE should be aseptically reconstituted only with the provided 2.0 mL of STERILE DILUENT (sterile water for injection). Sterile water diluent is not suitable for intravascular injection. This provides 2.5 mg melarsomine dihydrochloride per 0.1 mL of injectable solution. Two 50 mg vials will be required for dogs weighing >20 kg and 40 kg and 3 vials will be required for dogs >40 kg and 60 kg. Use immediately. Reconstituted solution may be used within 24 hours if refrigerated and kept from light.
Treatment Response: A baseline can be established pre-treatment by using commercially, available in-office heartworm antigen test kits prior to treatment. Treatment response can be assessed best by heartworm antigen testing applied 4 months after treatment. A successful treatment is determined to be conversion from an antigen positive to an antigen negative status. In dogs with signs of heartworm disease, gradual improvement should be observed as the long-term effects of the heartworm infection resolve. Some dogs may have chronic effects that will not totally resolve.
- CONCOMITANT THERAPY
- STORAGE CONDITIONS
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Carton
IMMITICIDE® Sterile Powder
(melarsomine dihydrochloride)Canine Heartworm Treatment
ACTIVE INGREDIENT: melarsomine dihydrochloride 5 x 50 mg vials
STERILE DILUENT (sterile water for injection) 5 x 2 ml vials
DILUENT for RECONSTITUTIONCAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
NADA 141-042, Approved by FDA
MERIAL
-
INGREDIENTS AND APPEARANCE
IMMITICIDE
melarsomine dihydrochloride and water kitProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 50604-2811 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50604-2811-1 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 VIAL, GLASS 10 mL Part 2 5 VIAL, GLASS 10 mL Part 1 of 2 MELARSOMINE DIHYDROCHLORIDE
melarsomine dihydrochloride injection, powder, lyophilized, for solutionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength melarsomine dihydrochloride (UNII: 9CVA716Q71) (melarsomine - UNII:374GJ0S41A) melarsomine dihydrochloride 50 mg in 2 mL Inactive Ingredients Ingredient Name Strength glycine (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141042 07/17/1995 Part 2 of 2 STERILE WATER
water for solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141042 07/17/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141042 07/17/1995 Labeler - Merial, Inc. (799641006)
Trademark Results [IMMITICIDE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMMITICIDE 73835197 1659379 Live/Registered |
RHONE MERIEUX 1989-10-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.