72937-181-02 72937-181-04 72937-181-08 72937-181-16 72937-181-40 72937-181-80
SUNSET PAIN RELIEF HEATING by
Drug Labeling and Warnings
SUNSET PAIN RELIEF HEATING by is a Otc medication manufactured, distributed, or labeled by SUNSET NOVELTIES, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
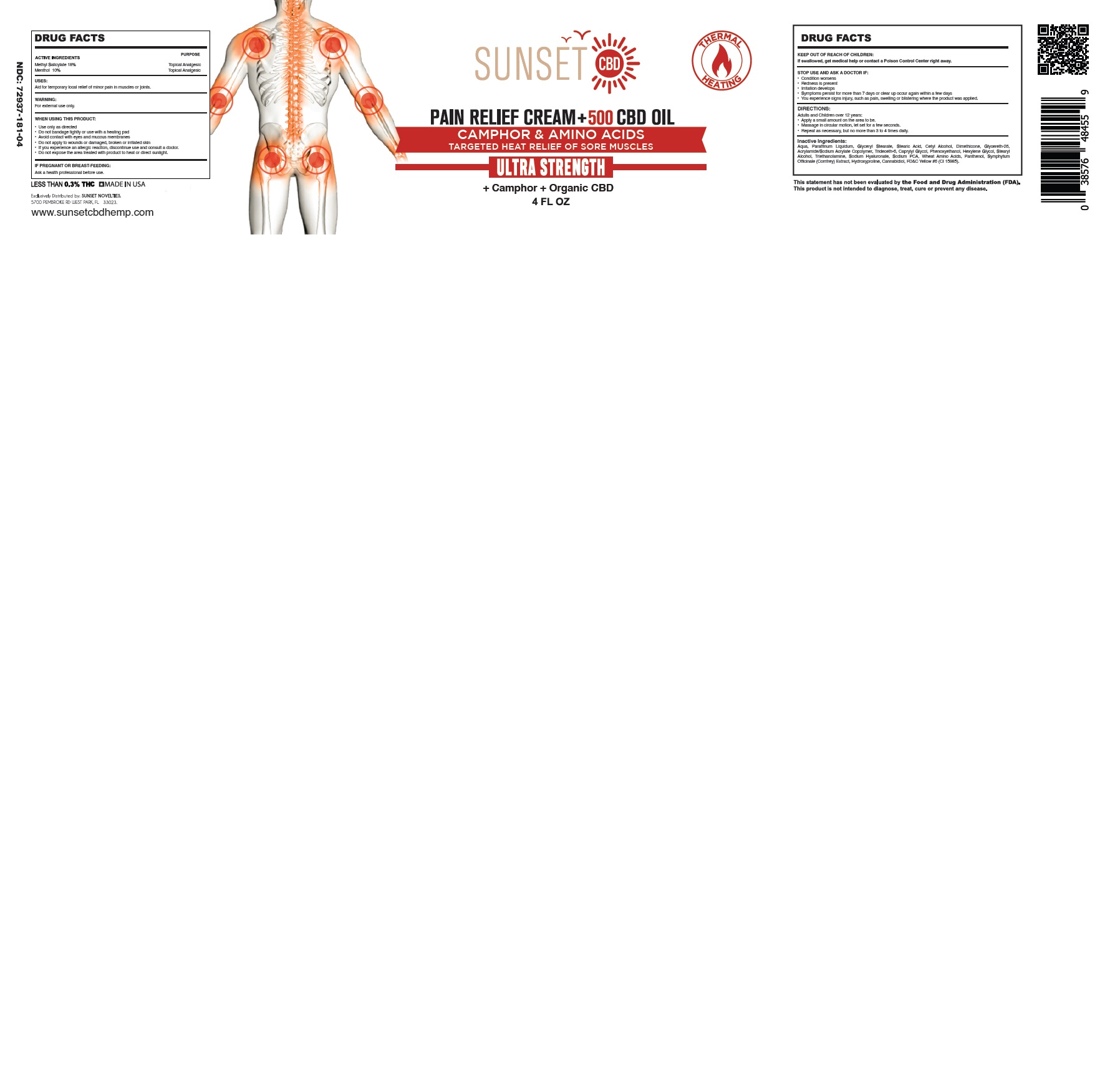
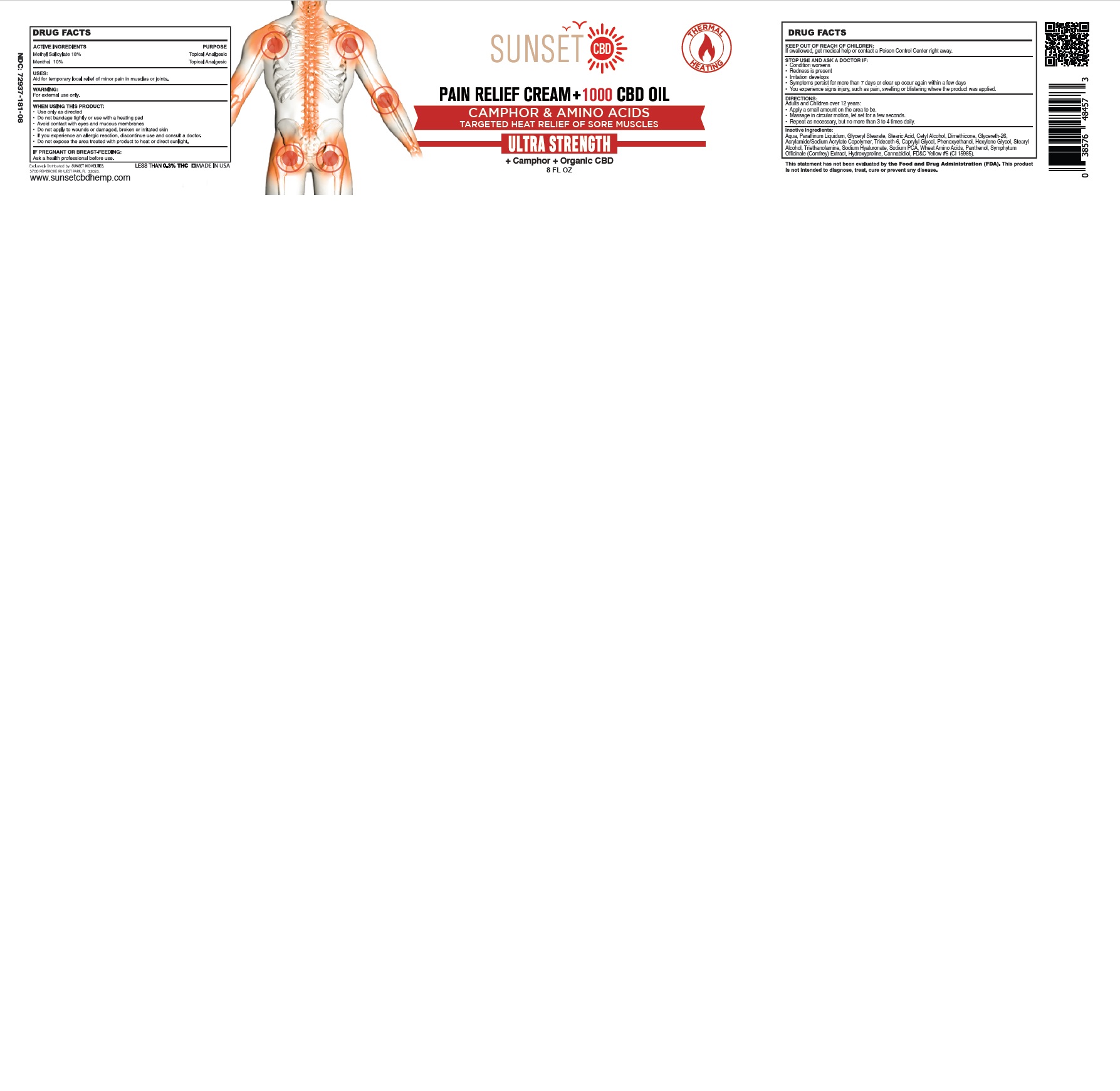
SUNSET PAIN RELIEF HEATING CAMPHOR AND AMINO ACIDS- methyl salicylate, menthol cream
SUNSET NOVELTIES, INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
72937-181-02
72937-181-04
72937-181-08
72937-181-16
72937-181-40
72937-181-80
Use only as directed
Do not bandage tightly or use with a heating pad
Avoid contact with eyes and mucous membranes
Do not apply to wounds or damaged, broken or irritated skin
If you experience an allergic reaction, discontinue use and consult a doctor.
Do not expose the area treated with product to heat or direct sunlight.
STOP USE AND ASK A DOCTOR IF:
Condition worsens
Redness is present
Irritation develops
Symptoms persist for more than 7 days or clear up occur again within a few days
You experience signs injury, such as pain, swelling or blistering where the product was applied.
DIRECTIONS:
Adults and Children over 12 years:
Apply a small amount on the area to be.
Massaged in a circular motion, until absorbed.
Repeat as necessary, but no more than 3 to 4 times daily.
Store tightly closed in a dry place at room temperature between 59°-86° F (15°-30° C).
Wash hands with soap and water after use.
Aqua, Paraffinum Liquidum, Glyceryl Stearate, Stearic Acid, Cetyl Alcohol, Dimethicone, Glycereth-26, Acrylamide/Sodium Acrylate Copolymer, Trideceth-6, Caprylyl Glycol, Phenoxyethanol, Hexylene Glycol, Stearyl Alcohol, Triethanolamine, Sodium Hyaluronate, Sodium PCA, Wheat Amino Acids, Panthenol, Symphytum Officinale (Comfrey) Extract, Hydroxyproline, Cannabidiol, FD&C Yellow #6 (CI 15985).
| SUNSET PAIN RELIEF HEATING
CAMPHOR AND AMINO ACIDS
methyl salicylate, menthol cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SUNSET NOVELTIES, INC (067218145) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.