TZIELD- teplizumab-mzwv injection
TZIELD by
Drug Labeling and Warnings
TZIELD by is a Prescription medication manufactured, distributed, or labeled by Provention Bio, Inc., AGC Biologics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TZIELD safely and effectively. See full prescribing information for TZIELD.
TZIELD® (teplizumab-mzwv) injection, for intravenous use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
TZIELD is a CD3-directed antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D (1).
DOSAGE AND ADMINISTRATION
- Confirm Stage 2 T1D by documenting at least two positive pancreatic islet autoantibodies in those who have dysglycemia without overt hyperglycemia using an oral glucose tolerance test (OGTT) or alternative method if appropriate and OGTT is not available (2.1).
- In patients who meet criteria for a diagnosis of Stage 2 type 1 diabetes, ensure the clinical history of the patient does not suggest type 2 diabetes (2.1).
- Prior to initiating TZIELD, obtain a complete blood count and liver enzyme tests. Use of TZIELD is not recommended in patients with certain laboratory abnormalities (2.2).
- Must dilute TZIELD in 0.9% Sodium Chloride Injection, USP. See full prescribing information for detailed preparation and administration instructions (2.3, 2.4, 2.5).
- Premedicate with: (1) a nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen, (2) an antihistamine, and/or (3) an antiemetic before each TZIELD dose for at least the first 5 days of the 14-day treatment course (2.3).
- Administer TZIELD by intravenous infusion (over a minimum of 30 minutes) once daily for 14 days. See full prescribing information for the dosing schedule (2.4).
DOSAGE FORMS AND STRENGTHS
- Injection: 2 mg per 2 mL (1 mg/mL) single-dose vial (3).
CONTRAINDICATIONS
- None. (4).
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): Premedicate, monitor liver enzymes, discontinue in those that develop elevated ALT or AST more than 5 times the upper limit of normal, and if severe CRS develops consider temporarily pausing dosing (5.1).
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection. Monitor for signs and symptoms of infection during and after TZIELD treatment. If a serious infection develops, discontinue TZIELD (5.2).
- Lymphopenia: Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia (<500 cells per mcL lasting 1 week or longer) develops, discontinue TZIELD (5.3).
- Hypersensitivity Reactions: If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly (5.4).
- Vaccinations: Administer all age-appropriate vaccinations prior to starting TZIELD. See recommendations regarding live-attenuated, inactivated, and mRNA vaccines (2.2, 5.5).
ADVERSE REACTIONS
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia and headache (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Provention Bio at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Laboratory Evaluation and Vaccination Prior to Initiation
2.3 Important Preparation and Premedication Instructions
2.4 Recommended Dosage and Administration
2.5 Additional Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Serious Infections
5.3 Lymphopenia
5.4 Hypersensitivity Reactions
5.5 Vaccinations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED / STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
TZIELD is indicated to delay the onset of Stage 3 type 1 diabetes in adults and pediatric patients 8 years of age and older with Stage 2 type 1 diabetes [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select adult patients and pediatric patients 8 years of age and older for TZIELD treatment who have a diagnosis of Stage 2 type 1 diabetes.
- Confirm Stage 2 type 1 diabetes by documenting:
- At least two positive pancreatic islet cell autoantibodies
- Dysglycemia without overt hyperglycemia using an oral glucose tolerance test (if an oral glucose tolerance test is not available, an alternative method for diagnosing dysglycemia without overt hyperglycemia may be appropriate)
- Ensure the clinical history of the patient does not suggest type 2 diabetes.
2.2 Laboratory Evaluation and Vaccination Prior to Initiation
- Prior to initiating TZIELD, obtain a complete blood count and liver enzyme tests.
- Use of TZIELD is not recommended in patients with [see Warnings and Precautions (5)]:
- Lymphocyte count less than 1,000 lymphocytes/mcL
- Hemoglobin less than 10 g/dL
- Platelet count less than 150,000 platelets/mcL
- Absolute neutrophil count less than 1,500 neutrophils/mcL
- Elevated ALT or AST greater than 2 times the upper limit of normal (ULN) or bilirubin greater than 1.5 times ULN
- Laboratory or clinical evidence of acute infection with Epstein-Barr virus (EBV) or cytomegalovirus (CMV)
- Active serious infection or chronic active infection other than localized skin infections
- Administer all age-appropriate vaccinations prior to starting TZIELD [see Warnings and Precautions (5.5)]:
- Administer live-attenuated (live) vaccines at least 8 weeks prior to treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment.
2.3 Important Preparation and Premedication Instructions
The following are important preparation and premedication instructions:
- Must dilute TZIELD prior to use [see Dosage and Administration (2.5)].
- Premedicate prior to TZIELD infusion for the first 5 days of dosing with: (1) a nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen, (2) an antihistamine, and/or (3) an antiemetic [see Warnings and Precautions (5.1)]. Administer additional doses of premedication if needed.
2.4 Recommended Dosage and Administration
Administer TZIELD by intravenous infusion (over a minimum of 30 minutes), using a body surface area-based dosing, once daily for 14 consecutive days as follows:
- Day 1: 65 mcg/m2
- Day 2: 125 mcg/m2
- Day 3: 250 mcg/m2
- Day 4: 500 mcg/m2
- Days 5 through 14: 1,030 mcg/m2
Do not administer two doses on the same day.
Recommendations Regarding Missed Dose(s)
If a planned TZIELD infusion is missed, resume dosing by administering all remaining doses on consecutive days to complete the 14-day treatment course.2.5 Additional Preparation and Administration Instructions
The following are additional preparation and administration instructions [see Dosage and Administration (2.2, 2.3, 2.4)]:
- Inspect TZIELD visually before use (the supplied solution is clear and colorless). Do not use TZIELD if particulate matter or coloration is seen.
- Prepare TZIELD using aseptic technique. Each vial is intended for single dose only.
- Prepare a:
- Sterile glass vial with 18 mL of 0.9% Sodium Chloride Injection or
- Polyvinylchloride (PVC) infusion bag with 18 mL of 0.9% Sodium Chloride Injection.
- Remove 2 mL of TZIELD from the vial and slowly add to the 18 mL of 0.9% Sodium Chloride Injection. Mix gently by slowly inverting the vial or rocking the infusion bag. The resulting 20 mL diluted solution contains 100 mcg/mL of teplizumab-mzwv.
- Using an appropriately sized syringe (e.g., 5 mL), withdraw the volume of diluted TZIELD solution required for that day's calculated dose from the 100 mcg/mL solution.
- Slowly add contents of the syringe containing the TZIELD dose to a 25 mL 0.9% Sodium Chloride Injection PVC infusion bag. Gently rock the infusion bag to ensure that the solution mixes sufficiently. Do not shake.
- Discard unused portion of remaining diluted TZIELD solution in the sterile glass vial or PVC infusion bag.
- Start the TZIELD infusion within 2 hours of preparation. If not used immediately, store the infusion solution at room temperature [15°C to 30°C (59°F to 86°F)] and complete infusion within 4 hours of the start of preparation. Discard the infusion solution if not administered within 4 hours of preparation.
- Confirm Stage 2 type 1 diabetes by documenting:
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
Cytokine release syndrome (CRS) has been observed in TZIELD-treated patients. In clinical trials, CRS was reported in 5% of TZIELD-treated patients compared to 0.8% of control-treated patients during the treatment period and through 28 days after the last study drug administration. CRS manifestations in TZIELD-treated patients included fever, nausea, fatigue, headache, myalgia, arthralgia, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), and increased total bilirubin. These manifestations typically occurred during the first 5 days of TZIELD treatment [see Adverse Reactions (6.1)]. To mitigate CRS:
- Premedicate with antipyretics, antihistamines and/or antiemetics prior to TZIELD treatment [see Dosage and Administration (2.3)].
- Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated ALT or AST more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Treat symptoms of CRS with antipyretics, antihistamines and/or antiemetics. If severe CRS develops, consider temporarily pausing dosing for 1-2 days (and administer the remaining doses to complete the full 14-day course on consecutive days) or discontinuing treatment.
5.2 Serious Infections
Bacterial and viral infections have occurred in TZIELD-treated patients. In clinical trials, TZIELD-treated patients had a higher rate of serious infections (3.5%) than control-treated patients (2%), including gastroenteritis, cellulitis, pneumonia, abscess, sepsis [see Adverse Reactions (6.1)]. Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD treatment. If serious infection develops, treat appropriately, and discontinue TZIELD.
5.3 Lymphopenia
In clinical trials, 78% of TZIELD-treated patients developed lymphopenia compared to 11% of control-treated patients. For most TZIELD-treated patients who experienced lymphopenia, lymphocyte levels began to recover after the fifth day of treatment and returned to pre-treatment values within two weeks after treatment completion and without dose interruption. Severe lymphopenia (<500 cells per mcL) lasting 1 week or longer occurred in 0.9% of TZIELD-treated patients, and 0.5% of TZIELD-treated patients permanently discontinued TZIELD because of lymphopenia [see Adverse Reactions (6.1)].
Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia (<500 cells per mcL lasting 1 week or longer) develops, discontinue TZIELD.
5.4 Hypersensitivity Reactions
Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients [see Adverse Reactions (6.1)]. If severe hypersensitivity reactions occur, discontinue use of TZIELD and treat promptly.
5.5 Vaccinations
The safety of immunization with live-attenuated vaccines in TZIELD-treated patients has not been studied. Additionally, TZIELD may interfere with the immune response to vaccination and decrease vaccine efficacy.
- Administer all age-appropriate vaccinations prior to starting TZIELD [see Dosage and Administration (2.2)].
- Inactivated or mRNA vaccinations are not recommended within the 2 weeks prior to TZIELD treatment, during treatment, or 6 weeks after completion of treatment.
- Live-attenuated vaccinations are not recommended within the 8 weeks prior to TZIELD treatment, during treatment, or up to 52 weeks after treatment.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the Prescribing Information:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Serious Infections [see Warnings and Precautions (5.2)]
- Lymphopenia [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Placebo-Controlled Study in Patients with Stage 2 Type 1 Diabetes
The data in Table 1 are derived from the placebo-controlled study (Study TN-10) in patients aged 8 years and older with Stage 2 type 1 diabetes (T1D) [see Clinical Studies (14)]. These data reflect exposure of 44 patients of whom 93% completed the full 14-day treatment course.Pool of Five Controlled Clinical Studies in Stage 2 Type 1 Diabetes and in an Unapproved Population
Adverse reactions in TZIELD-treated patients were also evaluated in a larger pool of adult and pediatric patients who participated in five controlled clinical studies (including Study TN-10 described above):
- One study in patients with Stage 2 T1D (Study TN-10) [see Clinical Studies (14)],
- Three placebo-controlled studies in an unapproved population,
- One open-label standard-of-care controlled study of TZIELD in an unapproved population.
In this pool:
- 773 patients received TZIELD (44 patients with Stage 2 TID and 729 patients from an unapproved population), and
- 245 patients received either placebo or standard of care control (32 patients with Stage 2 T1D and 213 patients from an unapproved population).
In these studies, 436 patients received a 14-day dosing regimen of TZIELD with a total drug exposure that was comparable to the total drug exposure achieved with the recommended dosage [see Dosage and Administration (2.4)], 168 patients received a 14-day course of TZIELD with a lower total TZIELD drug exposure, and 169 patients received a 6-day course of TZIELD with a lower total TZIELD drug exposure. The mean age of TZIELD-treated patients was 17.6 years (median 15 years), 62% were <18 years old (40% age 12 to 17; 21% age 8 to 11), and 64% were male. The population was 72% White, 26% Asian, 1% Black or African American, 1% were multiple or unknown race, and <1% American Indian or Alaska Native; 5% were Hispanic or Latino ethnicity.
Common Adverse Reactions
Table 1 presents common (≥ 5%) adverse reactions that occurred during treatment and through 28 days after the last study drug administration in Study TN-10. Adverse reactions observed in pediatric patients 8 years and older who received TZIELD were consistent with those reported in adult patients in this study.Table 1. Common Adverse Reactions* in Adult and Pediatric Patients Aged 8 Years and Older with Stage 2 Type 1 Diabetes (Study TN-10)† Adverse Reaction Placebo
N=32TZIELD
N=44- * That occurred during treatment and through 28 days after the last study drug administration
- † Adverse reactions that occurred in 2 or more TZIELD-treated patients
- ‡ Composite of rash-related terms including rash erythematous, rash macular, rash papular, rash maculo-papular, rash pruritic
Lymphopenia 6% 73% Rash‡ 0% 36% Leukopenia 0% 21% Headache 6% 11% Neutropenia 3% 5% Increased alanine aminotransferase 3% 5% Nausea 3% 5% Diarrhea 0% 5% Nasopharyngitis 0% 5% Cytokine Release Syndrome (CRS)
In Study TN-10, CRS was reported in 2% of TZIELD-treated patients compared to 0% of placebo-treated patients.Of the 39 TZIELD-treated patients that developed CRS (5% of all TZIELD-treated patients) in the pool of 5 clinical trials, 13% of the CRS cases were serious adverse reactions [see Warnings and Precautions (5.1)]. Liver transaminase elevations were observed in 56% of TZIELD-treated patients who experienced CRS: 64% were up to 2.5 times ULN, 32% were more than 2.5 to 5 times ULN, and 4.5% were 5-10 times ULN.
Serious Infections
In Study TN-10, serious infections (cellulitis, gastroenteritis, pneumonia, wound infection) were reported in 9% (4/44) of TZIELD-treated patients compared to 0% (0/32) of placebo-treated patients any time during or after the first dose of study treatment.Rash and Hypersensitivity Reactions
Hypersensitivity reactions were reported with TZIELD in Study TN-10. Serum sickness was observed in 2% (1/44) of TZIELD-treated patients compared to 0% (0/32) of placebo-treated patients. The patient who developed serum sickness had a prior history of positive anti-nuclear antibody and presented with arthralgias and elevated c-reactive protein and low C4 complement five days after completing their course of TZIELD; illness resolved in 2.5 months.In the pool of 5 clinical trials of patients:
- Anaphylaxis (with hypoxia and bronchospasm) was observed in one TZIELD-treated patient who was hospitalized.
- Angioedema (periorbital and facial) was observed in 0.3% TZIELD-treated patients, compared to 0% in control-treated patients. Peripheral and generalized edema was reported in 1.6% of TZIELD-treated patients and 0% of control-treated patients.
- Rash was observed in 48% of TZIELD-treated patients compared to 15% in control-treated patients, with 33 excess cases of rash per 100 patients. The majority of rashes observed with TZIELD treatment were not serious and resolved without intervention; although 0.3% (2/773) of TZIELD-treated patients had a serious rash compared to 0% (0/245) of placebo- treated patients.
- Urticaria was reported in 1.9% of TZIELD-treated patients and in 1.2% of control-treated patients.
Immunogenicity: Anti-Drug Antibody-Associated Adverse Reactions
In Study TN-10, rash occurred in 39% of TZIELD-treated patients who developed anti- teplizumab-mzwv antibodies and in 33% of TZIELD-treated patients who did not develop anti- teplizumab-mzwv antibodies [see Clinical Pharmacology (12.6)].Other Adverse Reactions
Lymphopenia
In Study TN-10, lymphopenia was reported in 73% of TZIELD-treated patients compared to 6% of placebo-treated patients. The average lymphocyte count nadir occurred at Day 5 of treatment, with recovery and return to baseline by Week 6 [see Warnings and Precautions (5.3)].Neutropenia
In Study TN-10, neutropenia was observed in 7% of TZIELD-treated patients compared to 3% of placebo-treated patients.Anemia and Thrombocytopenia
In the pool of 5 clinical trials of patients, anemia was reported in 27% of TZIELD-treated patients compared to 21% of placebo-treated patients, and thrombocytopenia was reported in 13% of TZIELD-treated patients compared to 5% of placebo-treated patients during the 14-day treatment course; recovery occurred within 2 to 4 weeks of treatment. In clinical trials, 1.8% of TZIELD-treated patients discontinued treatment due to hemoglobin less than 8.5 g/dL (or a decrease of more than 2 g/dL to a value less than 10 g/dL), and 1% discontinued TZIELD due to platelet count less than 50,000 platelets/mcL.Liver Enzyme Elevations
Liver enzyme elevations were observed in TZIELD-treated patients, both in the context of CRS and in patients without CRS. In the pool of 5 clinical trials, elevated aminotransferases were reported in 25% of TZIELD-treated patients compared to 11% of placebo-treated patients during the 14-day treatment course. On laboratory analysis, 5.1% of TZIELD-treated patients experienced a peak ALT more than 3 times the ULN compared to 0.8% of control-treated patients. Most liver enzyme elevations were transient and resolved 1-2 weeks after treatment; 98% resolved by follow-up week 14.Other Laboratory Abnormalities
In the pool of 5 clinical trials, other laboratory abnormalities including decreased bicarbonate (15% in TZIELD-treated patients, compared to 7% in placebo-treated patients) and decreased blood calcium (19% in TZIELD-treated patients, compared to 13% in placebo-treated patients) were noted. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available case reports from clinical trials with TZIELD are insufficient to identify a drug- associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Although there are no data on teplizumab-mzwv, monoclonal antibodies can be actively transported across the placenta, and TZIELD may cause immunosuppression in the utero- exposed infant (see Clinical Considerations). To minimize exposure to a fetus, avoid use of TZIELD during pregnancy and at least 30 days (6 half-lives) prior to planned pregnancy.TZIELD is not active in rodents. In animal reproduction studies, mice were given a surrogate anti-mouse CD3 antibody subcutaneously during organogenesis through lactation. Pups born to dams administered the murine surrogate antibody during pregnancy showed a reduction in the adaptive immune response consistent with the expected pharmacology (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Report pregnancies to Provention Bio, Inc.'s Adverse Event reporting line at 1-800-633-1610.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Transport of endogenous IgG antibodies across the placenta increases as pregnancy progresses, and peaks during the third trimester. Because teplizumab-mzwv may interfere with immune response to infections, risks and benefits should be considered prior to administering live vaccines to infants exposed to teplizumab-mzwv in utero. There are insufficient data regarding infant serum levels of teplizumab-mzwv at birth and the duration of persistence of teplizumab- mzwv in infant serum after birth to identify a specific timeframe to delay live virus immunizations in infants exposed in utero.Data
Animal Data
In an embryo-fetal developmental toxicity study, pregnant mice were administered a murine surrogate anti-mouse CD3 antibody by subcutaneous injection at dose levels of 0, 0.03, 0.3, or 20 mg/kg on Gestation Days 6, 10, and 14. Increase in post-implantation loss occurred in the 20 mg/kg group, in the presence of maternal toxicity.In a pre- and postnatal development toxicity study in pregnant mice, in which the murine surrogate antibody was administered every 3 days from gestation day 6 through lactation day 19 at doses of 0, 0.3, 3, or 20 mg/kg, no maternal toxicity or increased incidence of post- implantation loss was observed. Reductions in T cell populations and increases in B cells, and a reduction in the adaptive immune response to keyhole limpet hemocyanin (KLH) were observed in the offspring on postnatal days 35 and 84 at 20 mg/kg. The surrogate antibody was present in the offspring serum at level less than 1.5% that of maternal serum at the high dose. A trend towards reduction in fertility was observed in the offspring of dams administered the murine surrogate antibody at 20 mg/kg. The human relevance of this finding is unknown.
8.2 Lactation
Risk Summary
There are no data on the presence of teplizumab-mzwv in either human or animal milk, the effects on the breastfed child, or the effects on milk production. Endogenous maternal IgG and monoclonal antibodies are transferred into human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to teplizumab-mzwv are unknown.Although the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TZIELD and any potential adverse effects on the breastfed child from TZIELD or from the underlying maternal condition, a lactating woman may interrupt breastfeeding and pump and discard breast milk during treatment and for 20 days after TZIELD administration to minimize drug exposure to a breastfed child.
8.4 Pediatric Use
The safety and effectiveness of TZIELD to delay the onset of Stage 3 type 1 diabetes have been established in pediatric patients 8 years of age and older with Stage 2 type 1 diabetes. Use of TZIELD for this indication is supported by evidence from an adequate and well-controlled study (Study TN-10) in adults and pediatric patients 8 years of age and older (including 29 pediatric patients). Adverse reactions observed in pediatric patients 8 years of age and older who received TZIELD were consistent with those reported in adult patients [see Adverse Reactions (6.1)].
The safety and effectiveness of TZIELD have not been established in pediatric patients younger than 8 years of age.
-
11 DESCRIPTION
Teplizumab-mzwv is a CD3-directed monoclonal antibody (humanized IgG1 kappa) that has a molecular weight of approximately 150 kilodalton (kDa) and is expressed from a recombinant Chinese hamster ovary (CHO) cell line.
TZIELD (teplizumab-mzwv) injection is supplied as a sterile, preservative-free, clear and colorless solution in a 2 mg/2 mL (1 mg/mL) single-dose vial for intravenous use. Each mL contains 1 mg of teplizumab-mzwv, dibasic sodium phosphate (0.26 mg), monobasic sodium phosphate (0.98 mg), polysorbate 80 (0.05 mg), sodium chloride (8.78 mg), and water for injection. The pH is 6.1.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Teplizumab-mzwv binds to CD3 (a cell surface antigen present on T lymphocytes) and delays the onset of Stage 3 type 1 diabetes in adults and pediatric patients aged 8 years and older with Stage 2 type 1 diabetes. The mechanism may involve partial agonistic signaling and deactivation of pancreatic beta cell autoreactive T lymphocytes. Teplizumab-mzwv leads to an increase in the proportion of regulatory T cells and of exhausted CD8+ T cells in peripheral blood.
12.2 Pharmacodynamics
Clinical studies have shown that teplizumab-mzwv binds to CD3 molecules on the surface of both CD4+ and CD8+ T cells during treatment, with internalization of the teplizumab- mzwv/CD3 complex from the surface of T cells. Pharmacodynamic effects include lymphopenia in the absence of depletion of T cells with a nadir on the 5th day of dosing, during a 14-day course of TZIELD treatment [see Warnings and Precautions (5.3)]. Teplizumab-mzwv exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of teplizumab-mzwv have not been fully characterized.
12.3 Pharmacokinetics
Steady state concentrations of teplizumab-mzwv are not expected to be achieved during the 14- day course of TZIELD.
Distribution
The central volume of distribution (Vd) of teplizumab-mzwv was 2.27 L in a 60 kg subject.Elimination
Teplizumab-mzwv showed saturable binding and elimination. The mean (SD) terminal elimination half-life and clearance of teplizumab-mzwv are 4.5 (0.2) days and 2.7 (0.8) L/day in a 60 kg subject, respectively.Metabolism
Teplizumab-mzwv is expected to be metabolized into small peptides by catabolic pathways.Specific Populations
No clinically significant differences in the pharmacokinetics of teplizumab-mzwv were observed based on age (8 to 35 years old), biologic sex, or racial groups (White, Asians).BSA-based dosing normalizes the exposure to teplizumab-mzwv across body weight.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of teplizumab-mzwv or of other teplizumab products.
In the placebo-controlled study in patients aged 8 years of age and older with Stage 2 type 1 diabetes (Study TN-10) [see Clinical Studies (14)], approximately 57% of TZIELD-treated patients developed anti-teplizumab-mzwv antibodies, 46% of whom developed neutralizing antibodies. There is insufficient information to characterize the effects of ADA on pharmacokinetics, pharmacodynamics, or effectiveness of TZIELD. There was a higher incidence of rash in TZIELD-treated patients who developed anti-teplizumab-mzwv antibodies compared to those who did not develop anti-teplizumab-mzwv antibodies [see Adverse Reactions (6.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed to assess the carcinogenic potential of teplizumab- mzwv.
No studies have been performed to assess the mutagenic potential of teplizumab-mzwv. As an antibody, teplizumab-mzwv is not expected to interact directly with DNA.
Fertility and reproductive performance were unaffected in female and male mice that received a murine surrogate anti-mouse CD3 antibody administered by the subcutaneous route at doses up to 20 mg/kg.
-
14 CLINICAL STUDIES
The effectiveness of TZIELD was investigated in a randomized, double-blind, event-driven, placebo-controlled study (Study TN-10; NCT01030861) in 76 patients, 8 to 49 years of age with Stage 2 type 1 diabetes. Stage 2 type 1 diabetes was defined as having both of the following:
- Two or more of the following pancreatic islet autoantibodies:
- Glutamic acid decarboxylase 65 (GAD) autoantibodies
- Insulin autoantibody (IAA)
- Insulinoma-associated antigen 2 autoantibody (IA-2A)
- Zinc transporter 8 autoantibody (ZnT8A)
- Islet cell autoantibody (ICA)
- Dysglycemia on oral glucose tolerance testing
In this study, patients were randomized to receive TZIELD or placebo once daily by intravenous infusion for 14 days. Patients in the TZIELD group had a total drug exposure that was comparable to the total drug exposure achieved with the recommended total TZIELD dosage [see Dosage and Administration (2.4)]. The primary efficacy endpoint in this study was the time from randomization to development of Stage 3 type 1 diabetes diagnosis.
Baseline Patient Characteristics
In this study, 45% were female; 97% White, 1% Asian, and 1% reported multiracial background; 3% were Hispanic or Latino ethnicity; and 95% were from the United States. The median age was 14 years (72% were <18 years old) (Table 2).Table 2. Baseline Age Characteristics of Adults and Pediatric Patients 8 Years of Age and Older with Stage 2 Type 1 Diabetes (Study TN-10)* TZIELD
N=44Placebo
N=32- * Intent to treat (ITT) population
Age Group ≥ 18 Years 34% 19% < 18 years 66% 81% Pediatric Age Group Quartiles 8 to <11 years 21% 25% 11 to <14 years 27% 31% 14 to <18 years 18% 25% Baseline Disease Characteristics
Table 3 displays the baseline disease characteristics in Study TN-10.Table 3. Baseline Disease Characteristics of Adults and Pediatric Patients 8 Years of Age and Older with Stage 2 Type 1 Diabetes (Study TN-10)* TZIELD
N=44Placebo
N=32- * Intent to treat (ITT) population
- † The glucose data are area under the time-concentration curve (AUC) values from the oral glucose tolerance test
Glucose, mg/dL† median (min, max) 165 (115, 207) 154 (103, 200) HbA1c, % median (min, max) 5.2 (4.6, 6.1) 5.3 (4.3, 5.6) HLA-DR4 Missing 5% 0 Absent 34% 34% Present 61% 66% HLA-DR3 Missing 5% 0 Absent 48% 53% Present 48% 47% HLA-DR3/DR4 Both DR3 and DR4 25% 22% DR3 only 23% 25% DR4 only 36% 44% Missing 5% 0 Neither DR3 nor DR4 11% 9% Autoantibodies Positive (N) 1 2% 0 2 27% 22% 3 25% 16% 4 27% 44% 5 18% 19% Autoantibody Type Positive GAD65 91% 88% IAA 43% 34% IA-2A 59% 75% ICA 66% 88% ZnT8 73% 75% Abbreviations: HbA1c=hemoglobin A1c, SD=standard deviation, HLA = human leukocyte antigen, GAD65=Glutamic acid decarboxylase 65 (GAD) autoantibodies, IAA=Insulin autoantibody, IA- 2A=Insulinoma-associated antigen 2 autoantibody, ZnT8A=Zinc transporter 8 autoantibody, ICA=Islet cell autoantibody Efficacy Results
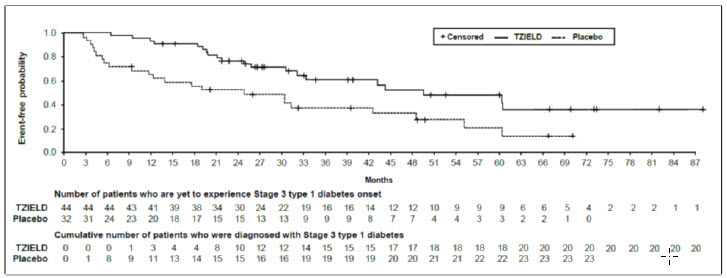
In Study TN-10, Stage 3 type 1 diabetes was diagnosed in 20 (45%) of the TZIELD-treated patients and in 23 (72%) of the placebo-treated patients. A Cox proportional hazards model, stratified by age and oral glucose tolerance test status at randomization, demonstrated that the median time from randomization to Stage 3 type 1 diabetes diagnosis was 50 months in the TZIELD group and 25 months in the placebo group, for a difference of 25 months. With a median follow-up time of 51 months, therapy with TZIELD resulted in a statistically significant delay in the development of Stage 3 type 1 diabetes, hazard ratio 0.41 (95% CI: 0.22 to 0.78; p=0.0066) (Figure 1).Study TN-10 was not designed to assess whether there were differences in the effectiveness between subgroups based on demographic characteristics or baseline disease characteristics.
Figure 1: Kaplan-Meier Curve of Time to Diagnosis of Stage 3 Type 1 Diabetes in Adult and Pediatric Patients Aged 8 Years and Older with Stage 2 Type 1 Diabetes by Treatment Group (Study TN-10)1
- 1 ITT population
- Two or more of the following pancreatic islet autoantibodies:
-
16 HOW SUPPLIED / STORAGE AND HANDLING
TZIELD (teplizumab-mzwv) injection is a clear and colorless solution (2 mg/2 mL (1 mg/mL)) supplied in a single-dose vial as follows:
Carton Contents NDC 1 single dose vial NDC: 73650-316-01 10 single dose vials NDC: 73650-316-10 14 single dose vials NDC: 73650-316-14 Refrigerate TZIELD vials at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Store upright. Do not freeze or shake the vials.
If not used immediately, store the diluted solution at room temperature [15°C to 30°C (59°F to 86°F)] and complete infusion within 4 hours of the start of preparation. Discard the diluted solution if not administered within 4 hours of preparation [see Dosage and Administration (2.5)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cytokine Release Syndrome
Inform patients about the signs and symptoms of CRS [see Warnings and Precautions (5.1)].Serious Infections
Inform patients that TZIELD may lower the ability of the immune system to fight infections. Instruct patients to contact their health care provider if they develop any symptoms of infection [see Warnings and Precautions (5.2)].Lymphopenia
Inform patients that although most TZIELD-treated patients had mild lymphopenia; a few had severe lymphopenia that required stopping TZIELD [see Warnings and Precautions (5.3)].Hypersensitivity Reactions
Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking TZIELD and seek medical attention promptly if such symptoms occur [see Warnings and Precautions (5.4)].Vaccinations
Advise patient to receive all age-appropriate vaccinations prior to starting TZIELD and avoid concurrent use of live, inactivated, and mRNA vaccines with TZIELD [see Warnings and Precautions (5.5)].Pregnancy
Advise patients to inform their health care provider of a known or suspected pregnancy. Advise patients who are exposed to TZIELD during pregnancy to contact Provention Bio, Inc.'s Adverse Event reporting line at 1-800-633-1610 [see Use in Specific Populations (8.1)].Lactation
Advise a lactating woman that she may interrupt breastfeeding and pump and discard breast milk during treatment and for 20 days after TZIELD administration to minimize drug exposure to a breastfed infant [see Use in Specific Populations (8.2)].Manufactured by:
Provention Bio, Inc.
Morristown, NJ 07960
A SANOFI COMPANY
U.S. License Number: 2170
TZIELD is a registered trademark of Provention Bio, Inc.
Copyright © 2025, Provention Bio, Inc. All rights reserved. -
MEDICATION GUIDE
MEDICATION GUIDE
TZIELD® (TEE-zeeld)
(teplizumab-mzwv)
injection, for intravenous useWhat is the most important information I should know about TZIELD?
TZIELD may cause serious side effects, including:
-
Cytokine Release Syndrome (CRS). Signs and symptoms of CRS problems may include:
- fever
- feeling tired (fatigue)
- muscle and joint pain
- nausea
- headache
- increased liver enzymes in your blood
- Decrease in white blood cells. TZIELD may cause a decrease in a type of white blood cell called lymphocytes. A decrease in white blood cells is a serious, but common side effect that can affect your body's ability to fight infections. A decrease in white blood cell counts can happen after your first dose. Your white blood cell counts will start to go back to normal after your fifth dose of TZIELD. Some people may develop longer and more severe decreases in lymphocytes.
See "What are the possible side effects of TZIELD?" for more information about side effects.What is TZIELD?
TZIELD is a prescription medicine used to delay the onset of Stage 3 type 1 diabetes, which is when your body can't make enough insulin on its own and may require insulin injections. TZIELD is for adults and children 8 years of age and older who have Stage 2 type 1 diabetes. This means that they have tested positive for 2 or more type 1 diabetes-related autoantibodies, have abnormal blood sugar levels and do not have type 2 diabetes.
It is not known if TZIELD is safe and effective in children under 8 years of age.Before or after receiving TZIELD, tell your healthcare provider about all your medical conditions, including if you: - have any of the conditions or symptoms listed in the section "What is the most important information I should know about TZIELD?"
- have a serious infection or an infection that does not go away or that keeps coming back (chronic).
- have recently received or are scheduled to receive an immunization (vaccine). TZIELD may affect how well a vaccine works. Tell your healthcare provider that you are receiving treatment with TZIELD before receiving a vaccine.
- are pregnant or plan to become pregnant. TZIELD may harm your unborn baby. Do not receive TZIELD during pregnancy and at least 30 days before a planned pregnancy.
- If you become pregnant while taking TZIELD, you are encouraged to report your pregnancy to the Provention Bio's Adverse Event reporting line at 1-800-633-1610.
- are breastfeeding or plan to breast feed. It is not known if TZIELD passes into your breast milk and if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you receive TZIELD. If you are breastfeeding, you may consider pumping and throwing away your breast milk during treatment with TZIELD and for 20 days after receiving TZIELD treatment.
How will I receive TZIELD?
- TZIELD is given by a healthcare provider through a needle placed in a vein (intravenous infusion) in your arm.
- You will receive a TZIELD infusion one-time a day, every day, for 14 days. Each TZIELD infusion will last about 30 minutes.
- For the first 5 days of treatment, your healthcare provider will give you medicines by mouth before starting your TZIELD infusion. These medicines include ibuprofen, naproxen or other pain relievers such as acetaminophen, an antihistamine, and an anti-nausea medicine. These medicines may help reduce symptoms of CRS such as a fever, headache, muscle and joint pain, or nausea.
- If you miss a scheduled infusion, your healthcare provider will continue your treatment on the next scheduled day. You will not receive 2 infusions on the same day.
What are the possible side effects of TZIELD?
TZIELD may cause serious side effects including:- See "What is the most important information I should know about TZIELD?"
- rash
- leukopenia (decrease in white blood cell counts)
- headache
General information about the safe and effective use of TZIELD.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about TZIELD that is written for health professionals.What are the ingredients in TZIELD?
Active ingredient: teplizumab-mzwv.
Inactive ingredients: dibasic sodium phosphate, monobasic sodium phosphate, polysorbate 80, sodium chloride, and water for injection.Manufactured by:
Provention Bio, Inc.
Morristown, NJ 07960
A SANOFI COMPANY
U.S. License Number: 2170
TZIELD is a registered trademark of Provention Bio, Inc.
Copyright © 2025, Provention Bio, Inc. All rights reserved.
For more information, call 1-800-633-1610 or go to www.tzield.com.This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 04/2025 -
Cytokine Release Syndrome (CRS). Signs and symptoms of CRS problems may include:
-
PRINCIPAL DISPLAY PANEL
Package Label - 1 count - 2 mg/2 mL Single-use VialPRINCIPAL DISPLAY PANEL
NDC: 73650-316-01
Rx only
Tzield™
(teplizumab-mzwv)
INJECTION
2 mg/2 mL (1 mg/1 mL)
Contains 1 single dose vial.
Discard unused portion.For intravenous infusion after dilution.
Store in the original carton
to protect from light.Dispense the accompanying
Medication Guide to each patient.proventionbio
-
PRINCIPAL DISPLAY PANEL
Package Label - 10 count - 2 mg/2 mL Single-use VialPRINCIPAL DISPLAY PANEL
NDC: 73650-316-10
Rx only
Tzield™
(teplizumab-mzwv)
INJECTION
2 mg/2 mL (1 mg/1 mL)
Contains 10 single dose vials.
Discard unused portion.For intravenous infusion after dilution.
Store in the original carton to protect
from light.Dispense the accompanying
Medication Guide to each patient.proventionbio
-
PRINCIPAL DISPLAY PANEL
Package Label - 14 count - 2 mg/2 mL Single-use VialPRINCIPAL DISPLAY PANEL
NDC: 73650-316-14
Rx only
Tzield™
(teplizumab-mzwv)
INJECTION
2 mg/2 mL (1 mg/1 mL)
Contains 14 single dose vials.
Discard unused portion.For intravenous infusion after dilution.
Store in the original carton to protect
from light.Dispense the accompanying
Medication Guide to each patient.proventionbio
-
INGREDIENTS AND APPEARANCE
TZIELD
teplizumab-mzwv injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73650-316 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TEPLIZUMAB (UNII: S4M959U2IJ) (TEPLIZUMAB - UNII:S4M959U2IJ) TEPLIZUMAB 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) 0.26 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 1.13 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.78 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.05 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73650-316-01 1 in 1 CARTON 11/17/2022 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 73650-316-10 10 in 1 CARTON 11/17/2022 2 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 3 NDC: 73650-316-14 14 in 1 CARTON 11/17/2022 3 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761183 11/17/2022 Labeler - Provention Bio, Inc. (080768105) Establishment Name Address ID/FEI Business Operations AGC Biologics, Inc. 824771724 API MANUFACTURE(73650-316) Establishment Name Address ID/FEI Business Operations Ajinomoto Althea, Inc. 023050730 MANUFACTURE(73650-316) Establishment Name Address ID/FEI Business Operations Almac Pharma Services LLC 078607239 LABEL(73650-316) , PACK(73650-316)
Trademark Results [TZIELD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TZIELD 90581915 not registered Live/Pending |
Provention Bio, Inc. 2021-03-16 |
 TZIELD 90085353 not registered Live/Pending |
PROVENTION BIO, INC. 2020-07-31 |
 TZIELD 88858541 not registered Live/Pending |
Provention Bio, Inc. 2020-04-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.