LOVAZA- omega-3-acid ethyl esters capsule, liquid filled
LOVAZA by
Drug Labeling and Warnings
LOVAZA by is a Prescription medication manufactured, distributed, or labeled by Atlantic Biologicals Corps. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LOVAZA safely and effectively. See full prescribing information for LOVAZA. LOVAZA (omega-3-acid ethyl esters) Capsules, for oral use Initial U.S. Approval: 2004
®
RECENT MAJOR CHANGES
Indications and Usage, Limitations of Use ( ) 1
05/2014
INDICATIONS AND USAGE
LOVAZA is a combination of ethyl esters of omega 3 fatty acids, principally EPA and DHA, indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia (HTG). ( ) 1
Limitations of Use:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 1-gram ( ) 3
CONTRAINDICATIONS
LOVAZA is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to LOVAZA or any of its components. ( ) 4
WARNINGS AND PRECAUTIONS
- In patients with hepatic impairment, monitor ALT and AST levels periodically during therapy. ( ) 5.1
- LOVAZA may increase levels of LDL. Monitor LDL levels periodically during therapy. ( ) 5.1
- Use with caution in patients with known hypersensitivity to fish and/or shellfish. ( ) 5.2
- There is a possible association between LOVAZA and more frequent recurrences of symptomatic atrial fibrillation or flutter in patients with paroxysmal or persistent atrial fibrillation, particularly within the first months of initiating therapy. ( ) 5.3
ADVERSE REACTIONS
The most common adverse reactions (incidence >3% and greater than placebo) were eructation, dyspepsia, and taste perversion. ( ) 6
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
Omega-3-acids may prolong bleeding time. Patients taking LOVAZA and an anticoagulant or other drug affecting coagulation (e.g., anti-platelet agents) should be monitored periodically. ( ) 7.1
USE IN SPECIFIC POPULATIONS
- Pregnancy: Use during pregnancy only if the potential benefit justifies the potential risk to the fetus. ( ) 8.1
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Monitoring: Laboratory Tests
5.3 Recurrent Atrial Fibrillation (AF) or Flutter
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anticoagulants or Other Drugs Affecting Coagulation
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Severe Hypertriglyceridemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
LOVAZA (omega-3-acid ethyl esters) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia (HTG). ®
Patients should be placed on an appropriate lipid-lowering diet before receiving LOVAZA and should continue this diet during treatment with LOVAZA. Usage Considerations:
Laboratory studies should be done to ascertain that the lipid levels are consistently abnormal before instituting therapy with LOVAZA. Every attempt should be made to control serum lipids with appropriate diet, exercise, weight loss in obese patients, and control of any medical problems such as diabetes mellitus and hypothyroidism that are contributing to the lipid abnormalities. Medications known to exacerbate hypertriglyceridemia (such as beta blockers, thiazides, estrogens) should be discontinued or changed if possible prior to consideration of triglyceride-lowering drug therapy.
Limitations of Use:
The effect of LOVAZA on the risk for pancreatitis has not been determined.
The effect of LOVAZA on cardiovascular mortality and morbidity has not been determined.
-
2 DOSAGE AND ADMINISTRATION
- Assess triglyceride levels carefully before initiating therapy. Identify other causes (e.g., diabetes mellitus, hypothyroidism, medications) of high triglyceride levels and manage as appropriate [see Indications and Usage (1)].
- Patients should be placed on an appropriate lipid-lowering diet before receiving LOVAZA, and should continue this diet during treatment with LOVAZA. In clinical studies, LOVAZA was administered with meals.
The daily dose of LOVAZA is 4 grams per day. The daily dose may be taken as a single 4-gram dose (4 capsules) or as two 2-gram doses (2 capsules given twice daily).
Patients should be advised to swallow LOVAZA capsules whole. Do not break open, crush, dissolve, or chew LOVAZA.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Monitoring: Laboratory Tests
In patients with hepatic impairment, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels should be monitored periodically during therapy with LOVAZA. In some patients, increases in ALT levels without a concurrent increase in AST levels were observed.
In some patients, LOVAZA increases LDL-C levels. LDL-C levels should be monitored periodically during therapy with LOVAZA.
Laboratory studies should be performed periodically to measure the patient’s TG levels during therapy with LOVAZA.
5.3 Recurrent Atrial Fibrillation (AF) or Flutter
In a double-blind, placebo-controlled trial of 663 subjects with symptomatic paroxysmal AF (n = 542) or persistent AF (n = 121), recurrent AF or flutter was observed in subjects randomized to LOVAZA who received 8 grams/day for 7 days and 4 grams/day thereafter for 23 weeks at a higher rate relative to placebo. Subjects in this trial had median baseline triglycerides of 127 mg/dL, had no substantial structural heart disease, were taking no anti-arrhythmic therapy (rate control permitted), and were in normal sinus rhythm at baseline.
At 24 weeks, in the paroxysmal AF stratum, there were 129 (47%) first recurrent symptomatic AF or flutter events on placebo and 141 (53%) on LOVAZA [primary endpoint, HR 1.19; 95% CI: 0.93, 1.35]. In the persistent AF stratum, there were 19 (35%) events on placebo and 34 (52%) events on LOVAZA [HR 1.63; 95% CI: 0.91, 2.18]. For both strata combined, the HR was 1.25; 95% CI: 1.00, 1.40. Although the clinical significance of these results is uncertain, there is a possible association between LOVAZA and more frequent recurrences of symptomatic atrial fibrillation or flutter in patients with paroxysmal or persistent atrial fibrillation, particularly within the first 2 to 3 months of initiating therapy.
LOVAZA is not indicated for the treatment of AF or flutter.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions reported in at least 3% and at a greater rate than placebo for subjects treated with LOVAZA based on pooled data across 23 clinical trials are listed in Table 1.
Table 1. Adverse Reactions Occurring at Incidence ≥3% and Greater than Placebo in Clinical Trials of LOVAZA Adverse Reaction a
LOVAZA
(N=655)
Placebo
(N=370)
n
%
n
%
Eructation
29
4
5
1
Dyspepsia
22
3
6
2
Taste perversion
27
4
1
<1
Trials included subjects with HTG and severe HTG. a
Additional adverse reactions from clinical trials are listed below:
Constipation, gastrointestinal disorder and vomiting. Digestive System:
Increased ALT and increased AST. Metabolic and Nutritional Disorders:
Pruritus and rash. Skin:
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the events described below have been identified during post-approval use of LOVAZA. Because these events are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or to always establish a causal relationship to drug exposure.
The following events have been reported: anaphylactic reaction, hemorrhagic diathesis.
-
7 DRUG INTERACTIONS
7.1 Anticoagulants or Other Drugs Affecting Coagulation
Some trials with omega-3-acids demonstrated prolongation of bleeding time. The prolongation of bleeding time reported in these trials has not exceeded normal limits and did not produce clinically significant bleeding episodes. Clinical trials have not been done to thoroughly examine the effect of LOVAZA and concomitant anticoagulants. Patients receiving treatment with LOVAZA and an anticoagulant or other drug affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. It is unknown whether LOVAZA can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. LOVAZA should be used during pregnancy only if the potential benefit to the patient justifies the potential risk to the fetus.
Animal Data:
Omega-3-acid ethyl esters have been shown to have an embryocidal effect in pregnant rats when given in doses resulting in exposures 7 times the recommended human dose of 4 grams/day based on a body surface area comparison.
In female rats given oral gavage doses of 100, 600, and 2,000 mg/kg/day beginning 2 weeks prior to mating and continuing through gestation and lactation, no adverse effects were observed in the high-dose group (5 times human systemic exposure following an oral dose of 4 grams/day based on body surface area comparison).
In pregnant rats given oral gavage doses of 1,000, 3,000, and 6,000 mg/kg/day from gestation day 6 through 15, no adverse effects were observed (14 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
In pregnant rats given oral gavage doses of 100, 600, and 2,000 mg/kg/day from gestation day 14 through lactation day 21, no adverse effects were seen at 2,000 mg/kg/day (5 times the human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison). However, decreased live births (20% reduction) and decreased survival to postnatal day 4 (40% reduction) were observed in a dose-ranging study using higher doses of 3,000 mg/kg/day (7 times the human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
In pregnant rabbits given oral gavage doses of 375, 750, and 1,500 mg/kg/day from gestation day 7 through 19, no findings were observed in the fetuses in groups given 375 mg/kg/day (2 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison). However, at higher doses, evidence of maternal toxicity was observed (4 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
8.3 Nursing Mothers
Studies with omega-3-acid ethyl esters have demonstrated excretion in human milk. The effect of this excretion on the infant of a nursing mother is unknown; caution should be exercised when LOVAZA is administered to a nursing mother. An animal study in lactating rats given oral gavage C-ethyl EPA demonstrated that drug levels were 6 to 14 times higher in milk than in plasma. 14
- 9 DRUG ABUSE AND DEPENDENCE
-
11 DESCRIPTION
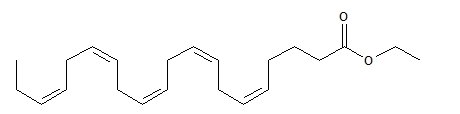
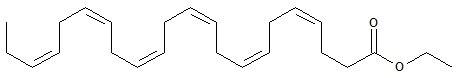
LOVAZA, a lipid-regulating agent, is supplied as a liquid-filled gel capsule for oral administration. Each 1-gram capsule of LOVAZA contains at least 900 mg of the ethyl esters of omega-3 fatty acids sourced from fish oils. These are predominantly a combination of ethyl esters of eicosapentaenoic acid (EPA - approximately 465 mg) and docosahexaenoic acid (DHA - approximately 375 mg).
The empirical formula of EPA ethyl ester is C H O , and the molecular weight of EPA ethyl ester is 330.51. The structural formula of EPA ethyl ester is: 22342
The empirical formula of DHA ethyl ester is C H O , and the molecular weight of DHA ethyl ester is 356.55. The structural formula of DHA ethyl ester is: 24362
LOVAZA capsules also contain the following inactive ingredients: 4 mg α-tocopherol (in a carrier of soybean oil), and gelatin, glycerol, and purified water (components of the capsule shell).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of LOVAZA is not completely understood. Potential mechanisms of action include inhibition of acyl-CoA:1,2-diacylglycerol acyltransferase, increased mitochondrial and peroxisomal β-oxidation in the liver, decreased lipogenesis in the liver, and increased plasma lipoprotein lipase activity. LOVAZA may reduce the synthesis of triglycerides in the liver because EPA and DHA are poor substrates for the enzymes responsible for TG synthesis, and EPA and DHA inhibit esterification of other fatty acids.
12.3 Pharmacokinetics
In healthy volunteers and in subjects with hypertriglyceridemia, EPA and DHA were absorbed when administered as ethyl esters orally. Omega-3-acids administered as ethyl esters (LOVAZA) induced significant, dose-dependent increases in serum phospholipid EPA content, though increases in DHA content were less marked and not dose-dependent when administered as ethyl esters.
Specific Populations:
Age:
Uptake of EPA and DHA into serum phospholipids in subjects treated with LOVAZA was independent of age (<49 years versus ≥49 years).
Drug-Drug Interactions:
Simvastatin:
In a 14-day trial of 24 healthy adult subjects, daily coadministration of simvastatin 80 mg with LOVAZA 4 grams did not affect the extent (AUC) or rate (C ) of exposure to simvastatin or the major active metabolite, beta-hydroxy simvastatin at steady state. max
Atorvastatin:
In a 14-day trial of 50 healthy adult subjects, daily coadministration of atorvastatin 80 mg with LOVAZA 4 grams did not affect AUC or C of exposure to atorvastatin, 2-hydroxyatorvastatin, or 4-hydroxyatorvastatin at steady state. max
Rosuvastatin:
In a 14-day trial of 48 healthy adult subjects, daily coadministration of rosuvastatin 40 mg with LOVAZA 4 grams did not affect AUC or C of exposure to rosuvastatin at steady state. max
In vitro studies using human liver microsomes indicated that clinically significant cytochrome P450-mediated inhibition by EPA/DHA combinations are not expected in humans.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a rat carcinogenicity study with oral gavage doses of 100, 600, and 2,000 mg/kg/day, males were treated with omega-3-acid ethyl esters for 101 weeks and females for 89 weeks without an increased incidence of tumors (up to 5 times human systemic exposures following an oral dose of 4 grams/day based on a body surface area comparison). Standard lifetime carcinogenicity bioassays were not conducted in mice.
Omega-3-acid ethyl esters were not mutagenic or clastogenic with or without metabolic activation in the bacterial mutagenesis (Ames) test with and or in the chromosomal aberration assay in Chinese hamster V79 lung cells or human lymphocytes. Omega-3-acid ethyl esters were negative in the in vivo mouse micronucleus assay. Salmonella typhimuriumEscherichia coli
In a rat fertility study with oral gavage doses of 100, 600, and 2,000 mg/kg/day, males were treated for 10 weeks prior to mating and females were treated for 2 weeks prior to and throughout mating, gestation, and lactation. No adverse effect on fertility was observed at 2,000 mg/kg/day (5 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
-
14 CLINICAL STUDIES
14.1 Severe Hypertriglyceridemia
The effects of LOVAZA 4 grams per day were assessed in 2 randomized, placebo-controlled, double-blind, parallel-group trials of 84 adult subjects (42 on LOVAZA, 42 on placebo) with very high triglyceride levels. Subjects whose baseline triglyceride levels were between 500 and 2,000 mg/dL were enrolled in these 2 trials of 6 and 16 weeks’ duration. The median triglyceride and LDL-C levels in these subjects were 792 mg/dL and 100 mg/dL, respectively. Median HDL-C level was 23.0 mg/dL.
The changes in the major lipoprotein lipid parameters for the groups receiving LOVAZA or placebo are shown in Table 2.
Table 2. Median Baseline and Percent Change From Baseline in Lipid Parameters in Subjects With Severe Hypertriglyceridemia (≥500 mg/dL) Parameter
LOVAZA
N=42
Placebo
N=42
Difference
BL
% Change
BL
% Change
TG
816
-44.9
788
+6.7
-51.6
Non-HDL-C
271
-13.8
292
-3.6
-10.2
TC
296
-9.7
314
-1.7
-8.0
VLDL-C
175
-41.7
175
-0.9
-40.8
HDL-C
22
+9.1
24
0.0
+9.1
LDL-C
89
+44.5
108
-4.8
+49.3
BL = Baseline (mg/dL); % Change = Median Percent Change from Baseline; Difference = LOVAZA Median % Change – Placebo Median % Change.
LOVAZA 4 grams per day reduced median TG, VLDL-C, and non-HDL-C levels and increased median HDL-C from baseline relative to placebo. Treatment with LOVAZA to reduce very high TG levels may result in elevations in LDL-C and non-HDL-C in some individuals. Patients should be monitored to ensure that the LDL-C level does not increase excessively.
The effect of LOVAZA on the risk of pancreatitis has not been determined.
The effect of LOVAZA on cardiovascular mortality and morbidity has not been determined.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC: 17856-0783-2 in a CUP of 1 CAPSULE, LIQUID FILLEDS
NDC: 17856-0783-9 in a POUCH of 1 CAPSULE, LIQUID FILLEDS
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Information for Patients:
- LOVAZA should be used with caution in patients with known sensitivity or allergy to fish and/or shellfish . [see Warnings and Precautions (5.2)]
- Advise patients that use of lipid-regulating agents does not reduce the importance of adhering to diet . [see Dosage and Administration (2)]
- Advise patients not to alter LOVAZA capsules in any way and to ingest intact capsules only . [see Dosage and Administration (2)]
- Instruct patients to take LOVAZA as prescribed. If a dose is missed, advise patients to take it as soon as they remember. However, if they miss one day of LOVAZA, they should not double the dose when they take it.
Manufactured for:
GlaxoSmithKline
Research Triangle Park, NC 27709
LOVAZA is a registered trademark of the GSK group of companies.
©2014, the GSK group of companies. All rights reserved.
LVZ:12PI
PHARMACIST-DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ __ _ _ _ _ _ _ _
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
LOVAZA (lō-vā-ză) ®
(omega-3-acid ethyl esters)
Capsules
Read this Patient Information before you start taking LOVAZA, and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is LOVAZA?
LOVAZA is a prescription medicine used along with a low fat and low cholesterol diet to lower very high triglyceride (fat) levels in adults.
It is not known if LOVAZA changes your risk of having inflammation of your pancreas (pancreatitis).
It is not known if LOVAZA prevents you from having a heart attack or stroke.
It is not known if LOVAZA is safe and effective in children.
Who should not take LOVAZA?
Do not take LOVAZA if you are allergic to omega-3-acid ethyl esters or any of the ingredients in LOVAZA. See the end of this leaflet for a complete list of ingredients in LOVAZA.
What should I tell my doctor before taking LOVAZA?
Before you take LOVAZA, tell your doctor if you:
- have diabetes.
- have a low thyroid problem (hypothyroidism).
- have a liver problem.
- have a pancreas problem.
- have a certain heart rhythm problem called atrial fibrillation or flutter.
- are allergic to fish or shellfish. It is not known if people who are allergic to fish or shellfish are also allergic to LOVAZA.
- are pregnant or plan to become pregnant. It is not known if LOVAZA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. LOVAZA can pass into your breast milk. You and your doctor should decide if you will take LOVAZA or breastfeed.
including prescription and non-prescription medicine, vitamins, and herbal supplements. Tell your doctor about all the medicines you take,
LOVAZA can interact with certain other medicines that you are taking. Using LOVAZA with medicines that affect blood clotting (anticoagulants or blood thinners) may cause serious side effects.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take LOVAZA?
- Take LOVAZA exactly as your doctor tells you to take it.
- You should not take more than 4 capsules of LOVAZA each day. Either take all 4 capsules at one time, or 2 capsules two times a day.
- Do not change your dose or stop LOVAZA without talking to your doctor.
- Take LOVAZA with or without food.
- Take LOVAZA capsules whole. Do not break, crush, dissolve, or chew LOVAZA capsules before swallowing. If you cannot swallow LOVAZA capsules whole, tell your doctor. You may need a different medicine.
- Your doctor may start you on a diet that is low in saturated fat, cholesterol, carbohydrates, and low in added sugars before giving you LOVAZA. Stay on this diet while taking LOVAZA.
- Your doctor should do blood tests to check your triglyceride, bad cholesterol and liver function levels while you take LOVAZA.
What are the possible side effects of LOVAZA?
LOVAZA may cause serious side effects, including:
- increases in the results of blood tests used to check your liver function (ALT and AST) and your bad cholesterol levels (LDL-C).
- increases in the frequency of a heart rhythm problem (atrial fibrillation or flutter) may especially happen in the first few months of taking LOVAZA if you already have that problem.
The most common side effects of LOVAZA include:
- burping
- upset stomach
- a change in your sense of taste.
Talk to your doctor if you have a side effect that bothers you or does not go away.
These are not all the possible side effects of LOVAZA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store LOVAZA?
- Store LOVAZA at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze LOVAZA.
- Safely throw away medicine that is out of date or no longer needed.
Keep LOVAZA and all medicines out of the reach of children.
General information about the safe and effective use of LOVAZA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LOVAZA for a condition for which it was not prescribed. Do not give LOVAZA to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information Leaflet summarizes the most important information about LOVAZA. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about LOVAZA that is written for health professionals.
For more information go to www.LOVAZA.com or call 1-888-825-5249.
What are the ingredients in LOVAZA?
Active Ingredient: omega-3-acid ethyl esters, mostly EPA and DHA
Inactive Ingredients: alpha-tocopherol (in soybean oil), gelatin, glycerol, purified water.
This patient labeling has been approved by the U.S. Food and Drug Administration.
Manufactured for:
GlaxoSmithKline
Research Triangle Park, NC 27709 LOVAZA is a registered trademark of the GSK group of companies.
©2014, the GSK group of companies. All rights reserved.
May 2014
LVZ:10PIL
- LOVAZA (OMEGA-3-ACID ETHYL ESTERS) CAPSULE, LIQUID FILLED
-
INGREDIENTS AND APPEARANCE
LOVAZA
omega-3-acid ethyl esters capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17856-0783(NDC:0173-0783) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEGA-3-ACID ETHYL ESTERS (UNII: D87YGH4Z0Q) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3-ACID ETHYL ESTERS 900 mg Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (light yellow) Score no score Shape CAPSULE Size 24mm Flavor Imprint Code LOVAZA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17856-0783-2 1 in 1 CUP; Type 0: Not a Combination Product 05/01/2015 2 NDC: 17856-0783-9 1 in 1 POUCH; Type 0: Not a Combination Product 05/03/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021654 10/21/2008 Labeler - Atlantic Biologicals Corps (047437707) Registrant - Atlantic Biologicals Corps (047437707) Establishment Name Address ID/FEI Business Operations Atlantic Biologicals Corps 047437707 RELABEL(17856-0783) , REPACK(17856-0783)
Trademark Results [LOVAZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LOVAZA 77189141 3423907 Live/Registered |
GLAXOSMITHKLINE LLC 2007-05-24 |
 LOVAZA 77139246 not registered Dead/Abandoned |
Reliant Pharmaceuticals, Inc. 2007-03-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.