PANCREAZE- pancrelipase lipase, pancrelipase amylase, and pancrelipase protease capsule, delayed release
PANCREAZE by
Drug Labeling and Warnings
PANCREAZE by is a Prescription medication manufactured, distributed, or labeled by VIVUS LLC, Nordmark Pharma GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PANCREAZE ® safely and effectively. See full prescribing information for PANCREAZE ®.
PANCREAZE ® (pancrelipase) delayed-release capsules
Initial U.S. Approval – 2010INDICATIONS AND USAGE
PANCREAZE ® is a combination of porcine-derived lipases, proteases, and amylases indicated for the treatment of exocrine pancreatic insufficiency due to cystic fibrosis or other conditions ( 1)
DOSAGE AND ADMINISTRATION
Dosage
PANCREAZE is not interchangeable with any other pancrelipase product.
Infants (up to 12 months)
- Infants may be given 2,600 lipase units per 120 mL of formula or per breast-feeding. ( 2.1)
- Do not mix PANCREAZE capsule contents directly into formula or breast milk prior to administration. ( 2.2)
Children Older than 12 Months and Younger than 4 Years
- Enzyme dosing should begin with 1,000 lipase units/kg of body weight per meal to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day. ( 2.1)
Children 4 Years and Older and Adults
- Enzyme dosing should begin with 500 lipase units/kg of body weight per meal to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day. ( 2.1)
Limitations on Dosing
- Dosing should not exceed the recommended maximum dosage set forth by the Cystic Fibrosis Foundation Consensus Conferences Guidelines. ( 2.1)
Administration
- PANCREAZE should be swallowed whole. For infants or patients unable to swallow intact capsules, the contents may be sprinkled on soft acidic food with a pH of 4.5 or less, e.g., applesauce. ( 2.2)
DOSAGE FORMS AND STRENGTHS
- Capsules: 2,600 USP units of lipase; 6,200 USP units of protease; 10,850 USP units of amylase. Capsules have a light orange opaque body and clear cap, printed with "VIVUS" and "MT 2"( 3)
- Capsules: 4,200 USP units of lipase; 14,200 USP units of protease; 24,600 USP units of amylase. Capsules have a yellow opaque body and clear cap, printed with "VIVUS" and "MT 4" ( 3)
- Capsules: 10,500 USP units of lipase; 35,500 USP units of protease; 61,500 USP units of amylase. Capsules have a pink opaque body and clear cap, printed with "VIVUS" and "MT 10" ( 3)
- Capsules: 16,800 USP units of lipase; 56,800 USP units of protease; 98,400 USP units of amylase. Capsules have a salmon opaque body and clear cap, printed with "VIVUS" and "MT 16" ( 3)
- Capsules: 21,000 USP units of lipase; 54,700 USP units of protease; 83,900 USP units of amylase. Capsules have a white opaque body and cap, printed with "VIVUS" and "MT 20" ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Fibrosing colonopathy is associated with high-dose use of pancreatic enzyme replacement. Exercise caution when doses of PANCREAZE exceed 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day). ( 5.1)
- To avoid irritation of oral mucosa, do not chew PANCREAZE or retain in the mouth. ( 5.2)
- Exercise caution when prescribing PANCREAZE to patients with gout, renal impairment, or hyperuricemia. ( 5.3)
- There is theoretical risk of viral transmission with all pancreatic enzyme products including PANCREAZE. ( 5.4)
- Exercise caution when administering pancrelipase to a patient with a known allergy to proteins of porcine origin. ( 5.5)
ADVERSE REACTIONS
- Treatment-emergent adverse events occurring in at least 2 patients (greater than or equal to 10%) receiving PANCREAZE or placebo are abdominal pain, abdominal pain upper, flatulence, diarrhea, abnormal feces, and fatigue. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact VIVUS, Inc., at 1-888-998-4887 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Pediatric Patients
- The safety and effectiveness of PANCREAZE were assessed in pediatric patients, aged 6 to 30 months old and aged 8 to 17 years old. ( 8.4)
- The safety and efficacy of pancreatic enzyme products with different formulations of pancrelipase in pediatric patients have been described in the medical literature and through clinical experience. ( 8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fibrosing Colonopathy
5.2 Potential for Irritation to Oral Mucosa
5.3 Potential for Risk of Hyperuricemia
5.4 Potential Viral Exposure from the Product Source
5.5 Allergic Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Dosing and Administration
17.2 Fibrosing Colonopathy
17.3 Allergic Reactions
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
PANCREAZE is not interchangeable with other pancrelipase products.PANCREAZE is not interchangeable with other pancrelipase products.
PANCREAZE is orally administered. Therapy should be initiated at the lowest recommended dose and gradually increased. The dosage of PANCREAZE should be individualized based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet (see below). PANCREAZE is orally administered. Therapy should be initiated at the lowest recommended dose and gradually increased. The dosage of PANCREAZE should be individualized based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet (see Limitations on Dosing below).
Dosage recommendations for pancreatic enzyme replacement therapy were published following the Cystic Fibrosis Foundation Consensus Conferences. 1,2,3 PANCREAZE should be administered in a manner consistent with the recommendations of the Conferences provided in the following paragraphs with one exception. The Conferences recommend doses of 2,000 to 4,000 lipase units in infants up to 12 months. PANCREAZE is available in a 2,600 lipase unit capsule. The recommended dose of PANCREAZE in infants up to 12 months is 2,600 lipase units per 120 mL of formula or per breast-feeding. Patients may be dosed on a fat ingestion-based or actual body weight-based dosing scheme.
Infants (up to 12 months)
Infants may be given 2,600 lipase units per 120 mL of formula or per breast-feeding. Do not mix PANCREAZE capsule contents directly into formula or breast milk prior to administration [see Dosage and Administration (2.2)] .
Children Older than 12 Months and Younger than 4 Years
Enzyme dosing should begin with 1,000 lipase units/kg of body weight per meal for children less than age 4 years to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day.
Children 4 Years and Older and Adults
Enzyme dosing should begin with 500 lipase units/kg of body weight per meal for those older than age 4 years to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day.
Usually, half of the prescribed PANCREAZE dose for an individualized full meal should be given with each snack. The total daily dose should reflect approximately three meals plus two or three snacks per day.
Enzyme doses expressed as lipase units/kg of body weight per meal should be decreased in older patients because they weigh more but tend to ingest less fat per kilogram of body weight.
Limitations on Dosing
Dosing should not exceed the recommended maximum dosage set forth by the Cystic Fibrosis Foundation Consensus Conferences Guidelines. 1, 2, 3
If symptoms and signs of steatorrhea persist, the dosage may be increased by a healthcare professional. Patients should be instructed not to increase the dosage on their own. There is great inter-individual variation in response to enzymes; thus, a range of doses is recommended. Changes in dosage may require an adjustment period of several days. If doses are to exceed 2,500 lipase units/kg of body weight per meal, further investigation is warranted.
Doses greater than 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day) should be used with caution and only if they are documented to be effective by 3-day fecal fat measures that indicate a significantly improved coefficient of fat absorption. Doses greater than 6,000 lipase units/kg of body weight per meal have been associated with colonic strictures, indicative of fibrosing colonopathy, in children with cystic fibrosis less than 12 years of age [see Warnings and Precautions (5.1)] . Patients currently receiving higher doses than 6,000 lipase units/kg of body weight per meal should be examined and the dosage either immediately decreased or titrated downward to a lower range.
2.2 Administration
PANCREAZE should always be taken as prescribed by a healthcare professional.
Infants (up to 12 months)
PANCREAZE should be administered to infants immediately prior to each feeding, using a dosage of 2,600 lipase units per 120 mL of formula or per breast-feeding (i.e., one capsule with 2,600 USP units of lipase). Contents of the capsule may be sprinkled on small amounts of acidic soft food with a pH of 4.5 or less (e.g., applesauce) and given to the infant within 15 minutes. Contents of the capsule may also be administered directly to the mouth. Administration should be followed by breast milk or formula. Contents of the capsule should not be mixed directly into formula or breast milk as this may diminish efficacy. Care should be taken to ensure that PANCREAZE is not crushed or chewed or retained in the mouth, to avoid irritation of the oral mucosa.
Children and Adults
PANCREAZE should be taken during meals or snacks, with sufficient fluid. PANCREAZE capsules and capsule contents should not be crushed or chewed. Capsules should be swallowed whole.
For patients who are unable to swallow intact capsules, the capsules may be carefully opened and the contents sprinkled on small amounts of acidic soft food with a pH of 4.5 or less (e.g., applesauce). The PANCREAZE-soft food mixture should be swallowed immediately without crushing or chewing, and followed with water or juice to ensure complete ingestion. Care should be taken to ensure that no drug is retained in the mouth.
-
3 DOSAGE FORMS AND STRENGTHS
The active ingredient in PANCREAZE evaluated in clinical trials is lipase. PANCREAZE is dosed by lipase units.
PANCREAZE is available in 5 color coded capsule strengths.
Other active ingredients include protease and amylase. Each PANCREAZE capsule strength contains the specified amounts of lipase, protease, and amylase as follows:
- 2,600 USP units of lipase; 6,200 USP units of protease; 10,850 USP units of amylase capsules have a light orange opaque body and clear cap, printed with "VIVUS" and "MT 2"
- 4,200 USP units of lipase; 14,200 USP units of protease; 24,600 USP units of amylase capsules have a yellow opaque body and clear cap, printed with "VIVUS" and "MT 4"
- 10,500 USP units of lipase; 35,500 USP units of protease; 61,500 USP units of amylase capsules have a pink opaque body and clear cap, printed with "VIVUS" and "MT 10"
- 16,800 USP units of lipase; 56,800 USP units of protease; 98,400 USP units of amylase capsules have a salmon opaque body and clear cap, printed with "VIVUS" and "MT 16"
- 21,000 USP units of lipase; 54,700 USP units of protease; 83,900 USP units of amylase capsules have a white opaque body and cap, printed with "VIVUS" and "MT 20"
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Fibrosing Colonopathy
Fibrosing colonopathy has been reported following treatment with different pancreatic enzyme products. 4,5 Fibrosing colonopathy is a rare serious adverse reaction initially described in association with high-dose pancreatic enzyme use, usually with use over a prolonged period of time and most commonly reported in pediatric patients with cystic fibrosis. The underlying mechanism of fibrosing colonopathy remains unknown. Doses of pancreatic enzyme products exceeding 6,000 lipase units/kg of body weight per meal have been associated with colonic strictures in children less than 12 years of age. 1 Patients with fibrosing colonopathy should be closely monitored because some patients may be at risk of progressing to stricture formation. It is uncertain whether regression of fibrosing colonopathy occurs. 1 It is generally recommended, unless clinically indicated, that enzyme doses should be less than 2,500 lipase units/kg of body weight per meal (or less than 10,000 lipase units/kg of body weight per day) or less than 4,000 lipase units/g fat ingested per day [see Dosage and Administration (2.1)].
Doses greater than 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day) should be used with caution and only if they are documented to be effective by 3-day fecal fat measures that indicate a significantly improved coefficient of fat absorption . Patients receiving higher doses than 6,000 lipase units/kg of body weight per meal should be examined and the dosage either immediately decreased or titrated downward to a lower range.
5.2 Potential for Irritation to Oral Mucosa
Care should be taken to ensure that no drug is retained in the mouth. PANCREAZE should not be crushed or chewed or mixed in foods having a pH greater than 4.5. These actions can disrupt the protective enteric coating resulting in early release of enzymes, irritation of oral mucosa, and/or loss of enzyme activity [see Dosage and Administration (2.2) and Patient Counseling Information (17)] . For patients who are unable to swallow intact capsules, the capsules may be carefully opened and the contents sprinkled to a small amount of acidic soft food with a pH of 4.5 or less, such as applesauce. The PANCREAZE-soft food mixture should be swallowed immediately and followed with water or juice to ensure complete ingestion.
5.3 Potential for Risk of Hyperuricemia
Caution should be exercised when prescribing PANCREAZE to patients with gout, renal impairment, or hyperuricemia. Porcine-derived pancreatic enzyme products contain purines that may increase blood uric acid levels.
5.4 Potential Viral Exposure from the Product Source
PANCREAZE is sourced from pancreatic tissue from swine used for food consumption. Although the risk that PANCREAZE will transmit an infectious agent to humans has been reduced by testing for certain viruses during manufacturing and by inactivating certain viruses during manufacturing, there is a theoretical risk for transmission of viral disease, including diseases caused by novel or unidentified viruses. Thus, the presence of porcine viruses that might infect humans cannot be definitely excluded. However, no cases of transmission of an infectious illness associated with the use of porcine pancreatic extracts have been reported.
5.5 Allergic Reactions
Caution should be exercised when administering pancrelipase to a patient with a known allergy to proteins of porcine origin. Rarely, severe allergic reactions including anaphylaxis, asthma, hives, and pruritus have been reported with other pancreatic enzyme products with different formulations of the same active ingredient (pancrelipase). The risks and benefits of continued PANCREAZE treatment in patients with severe allergy should be taken into consideration with the overall clinical needs of the patient.
-
6 ADVERSE REACTIONS
The most serious adverse reactions reported with different pancreatic enzyme products of the same active ingredient (pancrelipase) include fibrosing colonopathy, hyperuricemia and allergic reactions [see Warnings and Precautions (5)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The short-term safety of PANCREAZE was assessed in two clinical trials conducted in 57 patients with exocrine pancreatic insufficiency (EPI) due to CF. Study 1 was conducted in 40 patients, ages 8 years to 57 years; Study 2 was conducted in 17 patients, ages 6 months to 30 months. In Study 1, PANCREAZE was administered in a dose of approximately 6,300 lipase units per kilogram per day for lengths of treatment ranging from 8 to 26 days; in Study 2, PANCREAZE was administered in four treatment arms (doses of 1,375, 2,875, 4,735, and 5,938 lipase units per kilogram per day) for lengths of treatment ranging from 6 to 11 days. The population was nearly evenly distributed in gender, and approximately 96% of patients were Caucasian.
Study 1 was a randomized, double-blind, placebo-controlled study of 40 patients, ages 8 to 57 years, with EPI due to CF. In this study, patients received PANCREAZE at individually titrated doses (not to exceed 2,500 lipase units per kilogram per meal) for 14 days, followed by randomization to PANCREAZE or matching placebo for 7 days of treatment. The mean exposure to PANCREAZE during this study, including titration period and randomized withdrawal period, was 18 days.
The incidence of adverse events (regardless of causality) was higher during placebo treatment (60%) than during PANCREAZE treatment (40%). The most common adverse events reported during the study were gastrointestinal complaints, which were reported more commonly during placebo treatment (55%) than during PANCREAZE treatment (30%). The type and incidence of adverse events were similar in children (8 to 11 years), adolescents (12 to 17 years), and adults (greater than 18 years).
Table 1 enumerates treatment-emergent adverse events that occurred in at least 2 patients (greater than or equal to 10%) treated with either PANCREAZE or placebo in Study 1. Adverse events were classified by Medical Dictionary for Regulatory Activities (MedDRA) terminology.
Table 1. Treatment-Emergent Adverse Events Occurring in at Least 2 Patients (Greater Than or Equal to 10%) in Either Treatment Group of the Placebo-Controlled, Clinical Study of PANCREAZE MedDRA Primary System Organ Class
Preferred TermPANCREAZE
(N=20)
n (%)Placebo
(N=20)
n (%)Gastrointestinal Disorders Abdominal pain 2 (10%) 3 (15%) Abdominal pain upper 1 (5%) 3 (15%) Flatulence 1 (5%) 3 (15%) Diarrhea 0 (0%) 4 (20%) Abnormal feces 0 (0%) 3 (15%) General Disorders and Administration Site Conditions Fatigue 0 (0%) 2 (10%) Study 2 was a randomized, investigator-blinded, dose-ranging study of 17 patients, ages 6 months to 30 months, with EPI due to CF. All patients were transitioned from their usual PEP treatment to PANCREAZE at 375 lipase units per kilogram body weight per meal for a 6 day run-in period. Patients were then randomized to receive PANCREAZE at one of four doses (375, 750, 1,125, and 1,500 lipase units per kilogram body weight per meal) for 5 days. Adverse events were collected on patient diary entries and at each study visit.
The most commonly reported adverse events were gastrointestinal, including diarrhea and vomiting, and were similar in type and frequency across treatment arms and to those reported in the double-blind, placebo-controlled trial (Study 1).
6.2 Postmarketing Experience
Postmarketing data for PANCREAZE have been available since 1988. The safety data are similar to those described below.
Delayed- and immediate-release pancreatic enzyme products with different formulations of the same active ingredient (pancrelipase) have been used for the treatment of patients with exocrine pancreatic insufficiency due to cystic fibrosis and other conditions, such as chronic pancreatitis. The long-term safety profile of these products has been described in the medical literature. The most serious adverse events included fibrosing colonopathy, distal intestinal obstruction syndrome (DIOS), recurrence of pre-existing carcinoma, and severe allergic reactions including anaphylaxis, asthma, hives, and pruritus. The most commonly reported adverse events were gastrointestinal disorders, including abdominal pain, diarrhea, flatulence, constipation and nausea, and skin disorders including pruritus, urticaria and rash. In general, these products have a well-defined and favorable risk-benefit profile in exocrine pancreatic insufficiency.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects
Pregnancy Category C: Animal reproduction studies have not been conducted with pancrelipase. It is not known whether pancrelipase can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. PANCREAZE should be given to a pregnant woman only if clearly needed. The risk and benefit of pancrelipase should be considered in the context of the need to provide adequate nutritional support to a pregnant woman with exocrine pancreatic insufficiency. Adequate caloric intake during pregnancy is important for normal maternal weight gain and fetal growth. Reduced maternal weight gain and malnutrition can be associated with adverse pregnancy outcomes.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PANCREAZE is administered to a nursing woman. The risk and benefit of pancrelipase should be considered in the context of the need to provide adequate nutritional support to a nursing mother with exocrine pancreatic insufficiency.
8.4 Pediatric Use
The short-term safety and effectiveness of PANCREAZE were assessed in two clinical studies in pediatric patients with EPI due to CF; one study included patients ages 6 to 30 months, and the other included patients ages 8 years to 17 years.
Study 1 was a randomized, double-blind, placebo-controlled study in 40 patients, 14 of whom were pediatric patients, including 7 children aged 8 to 11 years, and 7 adolescents aged 12 to 17 years. The safety and efficacy in pediatric patients in this study were similar to adult patients [see Adverse Reactions (6.1) and Clinical Studies (14)] .
Study 2 was a randomized, investigator-blinded, dose-ranging study in 17 pediatric patients aged 6 to 30 months. When patient regimen was switched from their usual PEP regimen to PANCREAZE, patients showed similar control of their fat malabsorption [see Adverse Reactions (6.1) and Clinical Studies (14)] .
The safety and efficacy of pancreatic enzyme products with different formulations of pancrelipase consisting of the same active ingredients (lipases, proteases, and amylases) for treatment of children with exocrine pancreatic insufficiency due to cystic fibrosis has been described in the medical literature and through clinical experience.
Dosing of pediatric patients should be in accordance with recommended guidance from the Cystic Fibrosis Foundation Consensus Conferences [see Dosage and Administration (2.1)]. Doses of other pancreatic enzyme products exceeding 6,000 lipase units/kg of body weight per meal have been associated with fibrosing colonopathy and colonic strictures in children less than 12 years of age [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
In Study 1, a 10 year-old patient was administered a PANCREAZE dose of 12,399 lipase units per kilogram per day for the duration of the open-label and randomized withdrawal periods. The patient experienced mild abdominal pain throughout both study periods. Abnormal chemistry data at the end of the study included mild elevations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum phosphate. Abnormal hematology data at the end of the study included mild elevations of hematocrit. No abnormalities from analyses of urinalysis or uric acid were noted.
Chronic high doses of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)] . High doses of pancreatic enzyme products have been associated with hyperuricosuria and hyperuricemia, and should be used with caution in patients with a history of hyperuricemia, gout, or renal impairment [see Warnings and Precautions (5.3)] .
-
11 DESCRIPTION
PANCREAZE is a pancreatic enzyme preparation consisting of pancrelipase, an extract derived from porcine pancreatic glands. Pancrelipase contains multiple enzyme classes, including porcine-derived lipases, proteases, and amylases.
Each capsule for oral administration contains enteric-coated microtablets that are each approximately 2 mm in diameter.
The active ingredient evaluated in clinical trials is lipase. PANCREAZE is dosed by lipase units. Other active ingredients include protease and amylase.
Inactive ingredients in PANCREAZE 2,600 USP units of lipase include colloidal silicon dioxide, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, microcrystalline cellulose, montan glycol wax, simethicone emulsion, talc and triethyl citrate.
Inactive ingredients in other strengths of PANCREAZE include cellulose, colloidal anhydrous silica, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, montan glycol wax, simethicone emulsion, talc and triethyl citrate.
PANCREAZE is available in five color coded strengths. Each PANCREAZE capsule strength contains the specified amounts of lipase, protease, and amylase as follows:
2,600 USP units of lipase; 6,200 USP units of protease; 10,850 USP units of amylase. The hypromellose capsules have a light orange opaque body and clear cap imprinted with "VIVUS" and "MT 2". The capsule shell contains hypromellose, titanium dioxide, iron oxide, and imprint ink contains iron oxide, shellac, ammonium hydroxide, propylene glycol, potassium hydroxide.
4,200 USP units of lipase; 14,200 USP units of protease; 24,600 USP units of amylase. The hard gelatin capsules have a yellow opaque body and clear cap imprinted with "VIVUS" and "MT 4". The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, sorbitan monolaurate, iron oxide, and gelatin capsule imprint ink.
10,500 USP units of lipase; 35,500 USP units of protease; 61,500 USP units of amylase. The hard gelatin capsules have a pink opaque body and clear cap imprinted with "VIVUS" and "MT 10". The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, sorbitan monolaurate, iron oxide, and gelatin capsule imprint ink.
16,800 USP units of lipase; 56,800 USP units of protease; 98,400 USP units of amylase. The hard gelatin capsules have a salmon opaque body and clear cap imprinted with "VIVUS" and "MT 16". The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, sorbitan monolaurate, iron oxide, and gelatin capsule imprint ink.
21,000 USP units of lipase; 54,700 USP units of protease; 83,900 USP units of amylase. The hard gelatin capsules have a white opaque body and cap imprinted with "VIVUS" and "MT 20". The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, sorbitan monolaurate, and gelatin capsule imprint ink.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The pancreatic enzymes in PANCREAZE catalyze the hydrolysis of fats to monoglyceride, glycerol and free fatty acids, proteins into peptides and amino acids, and starches into dextrins and short chain sugars such as maltose and maltriose in the duodenum and proximal small intestine, thereby acting like digestive enzymes physiologically secreted by the pancreas.
12.3 Pharmacokinetics
The pancreatic enzymes in PANCREAZE are enteric-coated to minimize destruction or inactivation in gastric acid. PANCREAZE is expected to release most of the enzymes in vivo at pH greater than 5.5. Pancreatic enzymes are not absorbed from the gastrointestinal tract in appreciable amounts.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The short-term safety and efficacy of PANCREAZE were evaluated in two studies conducted in 57 patients with exocrine pancreatic insufficiency (EPI) associated with cystic fibrosis (CF).
Study 1 was a randomized, double-blind, placebo-controlled study of 40 patients, ages 8 to 57 years, with EPI due to CF. In this study, patients received PANCREAZE at individually titrated doses (not to exceed 2,500 lipase units per kilogram per meal) for 14 days (open-label period) followed by randomization to PANCREAZE or matching placebo for 7 days of treatment (double-blind withdrawal period). Only patients with coefficient of fat absorption (CFA) ≥80% in the open-label period were randomized to the double-blind withdrawal period. The mean dose during the controlled treatment period was 6,400 lipase units per kilogram per day. All patients consumed a high-fat diet (greater than or equal to 100 grams of fat per day) during the treatment period.
The primary efficacy endpoint was the change in CFA from the open label period to the end of the double-blind withdrawal period. The CFA was determined by a 72-hour stool collection period during both treatment periods, when both fat excretion and fat ingestion were measured (Table 2).
Table 2. Change in CFA in Study 1 (Open-Label Period to End of Double-Blind Withdrawal Period) PANCREAZE
n=20Placebo
n=20- * Minimum of 72 hours from start of open label period.
- † Double-blind withdrawal period ranged from 4 to 7 days.
- ‡ p<0.001
CFA [%] Open-Label Period * (Mean, SD) 88 (5) 91 (5) End of Double-Blind Withdrawal Period † (Mean, SD) 87 (8) 56 (25) Change in CFA ‡ [%] Open-Label Period to End of Double-Blind Withdrawal Period (Mean, SD) -2 (6) -34 (23) Treatment Difference Point Estimate (95% CI) 33 (25, 40) At the end of the double-blind withdrawal period, the mean change in CFA from the open-label period to the end of the double-blind withdrawal period was -2% with PANCREAZE treatment compared to -34% with placebo treatment. There were similar responses to PANCREAZE by age and gender.
Study 2 was a randomized, investigator-blinded, dose-ranging study of 17 patients, ages 6 months to 30 months (mean 18 months) with EPI due to CF. The final analysis population was limited to 16 patients; 1 patient was excluded due to withdrawal of consent. All patients were transitioned from their usual PEP treatment to PANCREAZE at 375 lipase units per kilogram body weight per meal for a 6-day run-in period. Patients were then randomized to receive PANCREAZE at one of four doses (375, 750, 1,125, and 1,500 lipase units per kilogram body weight per meal) for 5 days. The CFA was measured at the end of the run-in period and at the end of the randomized period (Table 3).
Table 3. Change in CFA in Study 2 (End of Run-in Period to End of Study) 375 units
lipase/kg/meal
n=4750 units
lipase/kg/meal
n=41,125 units
lipase/kg/meal
n=41,500 units
lipase/kg/meal
n=4- * End of Run-in Period;
- † End of Study
CFA (%) Day 6 * (Mean, SD) 93 (2) 90 (5) 81 (11) 93 (3) Day 11 † (Mean, SD) 92 (3) 91 (4) 80 (13) 91 (2) Change in CFA (%) Day 6 to Day 11 (Mean, SD) -2 (3) 1 (3) -1 (3) -2 (3) Overall, patients showed similar CFA at the end of the run-in period (mean PANCREAZE dose of 1,600 lipase units per kilogram body weight per day) as at the end of the study across the four treatment arms.
-
15 REFERENCES
- Borowitz DS, Grand RJ, Durie PR, et al. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Journal of Pediatrics. 1995; 127: 681-684.
- Borowitz DS, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. Journal of Pediatric Gastroenterology Nutrition. 2002 Sep; 35: 246-259.
- Stallings VA, Start LJ, Robinson KA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. Journal of the American Dietetic Association. 2008; 108: 832-839.
- Smyth RL, Ashby D, O'Hea U, et al. Fibrosing colonopathy in cystic fibrosis: results of a case-control study. Lancet. 1995; 346: 1247-1251.
- FitzSimmons SC, Burkhart GA, Borowitz DS, et al. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. New England Journal of Medicine. 1997; 336: 1283-1289.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PANCREAZE (pancrelipase) Delayed-Release Capsules
2,600 USP units of lipase; 6,200 USP units of protease; 10,850 USP units of amylase.
PANCREAZE (pancrelipase) is supplied as hypromellose capsules with a light orange opaque body and clear cap imprinted with "VIVUS" and "MT 2" and packaged in bottles of 100-(NDC: 62541-401-10).
PANCREAZE (pancrelipase) Delayed-Release Capsules
4,200 USP units of lipase; 14,200 USP units of protease; 24,600 USP units of amylase.
PANCREAZE (pancrelipase) is supplied as hard gelatin capsules with a yellow opaque body and clear cap imprinted with "VIVUS" and "MT 4" and packaged in bottles of 100-(NDC: 62541-402-10).
PANCREAZE (pancrelipase) Delayed-Release Capsules
10,500 USP units of lipase; 35,500 USP units of protease; 61,500 USP units of amylase.
PANCREAZE (pancrelipase) is supplied as hard gelatin capsules with a pink opaque body and clear cap imprinted with "VIVUS" and "MT 10" and packaged in bottles of 100-(NDC: 62541-403-10).
PANCREAZE (pancrelipase) Delayed-Release Capsules
16,800 USP units of lipase; 56,800 USP units of protease; 98,400 USP units of amylase.
PANCREAZE (pancrelipase) is supplied as hard gelatin capsules with a salmon opaque body and clear cap imprinted with "VIVUS" and "MT 16" and packaged in bottles of 100-(NDC: 62541-404-10).
PANCREAZE (pancrelipase) Delayed-Release Capsules
21,000 USP units of lipase; 54,700 USP units of protease; 83,900 USP units of amylase.
PANCREAZE (pancrelipase) is supplied as hard gelatin capsules with a white opaque body and cap imprinted with "VIVUS" and "MT 20" and packaged in bottles of 100-(NDC: 62541-405-10).
Storage and Handling
Avoid heat. PANCREAZE capsules should be stored in a dry place in the original container. After opening, KEEP THE CONTAINER TIGHTLY CLOSED between uses to PROTECT FROM MOISTURE. Do not store above 25°C (77°F).
The PANCREAZE 2,600 USP units of lipase, the PANCREAZE 4,200 USP units of lipase and the PANCREAZE 21,000 USP units of lipase bottles contain a desiccant canister. Do not eat or throw away the desiccant canister in your medicine bottle. This canister will protect your medicine from moisture.
Keep out of reach of children.
DO NOT CRUSH PANCREAZE delayed-release capsules or the capsule contents.
-
17 PATIENT COUNSELING INFORMATION
See Medication Guide
17.1 Dosing and Administration
Instruct patients and caregivers that PANCREAZE should only be taken as directed by their healthcare professional. Patients should be advised that the total daily dose should not exceed 10,000 lipase units/kg body weight/day unless clinically indicated. This needs to be especially emphasized for patients eating multiple snacks and meals per day. Patients should be informed that if a dose is missed, the next dose should be taken with the next meal or snack as directed. Doses should not be doubled [see Dosage and Administration (2)].
Instruct patients and caregivers that PANCREAZE should always be taken with food. Patients should be advised that PANCREAZE delayed-release capsules and the capsule contents must not be crushed or chewed as doing so could cause early release of enzymes and/or loss of enzymatic activity. Patients should swallow the intact capsules with adequate amounts of liquid at mealtimes. If necessary, the capsule contents can also be sprinkled on soft acidic foods. [see Dosage and Administration (2)] .
Instruct patients to notify their healthcare professional if they are pregnant or are thinking of becoming pregnant during treatment with PANCREAZE [see Use in Specific Populations (8.1)].
Instruct patients to notify their healthcare professional if they are breastfeeding or are thinking of breastfeeding during treatment with PANCREAZE [see Use in Specific Populations (8.3)].
17.2 Fibrosing Colonopathy
Advise patients and caregivers to follow dosing instructions carefully, as doses of pancreatic enzyme products exceeding 6,000 lipase units/kg of body weight per meal (10,000 lipase units/kg of body weight/day) have been associated with colonic strictures in children below the age of 12 years [see Dosage and Administration (2)] .
17.3 Allergic Reactions
Advise patients and caregivers to contact their healthcare professional immediately if allergic reactions to PANCREAZE develop [see Warnings and Precautions (5.5)] .
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
PANCREAZE ® (pan-kre-aze)
(pancrelipase)
delayed-release capsulesRead this Medication Guide before you start taking PANCREAZE and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
What is the most important information I should know about PANCREAZE?
PANCREAZE may increase your chance of having a rare bowel disorder called fibrosing colonopathy. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gave you.
Call your doctor right away if you have any unusual or severe:
- stomach area (abdominal) pain
- bloating
- trouble passing stool (having bowel movements)
- nausea, vomiting, or diarrhea
Take PANCREAZE exactly as prescribed by your doctor. Do not take more or less PANCREAZE than directed by your doctor.
What is PANCREAZE?
PANCREAZE is a prescription medicine used to treat people who cannot digest food normally because their pancreas does not make enough enzymes due to cystic fibrosis or other conditions. PANCREAZE may help your body use fats, proteins, and sugars from food.
PANCREAZE contains a mixture of digestive enzymes including lipases, proteases, and amylases from pig pancreas.
PANCREAZE is safe and effective in children when taken as prescribed by your doctor.
What should I tell my doctor before taking PANCREAZE?
Before taking PANCREAZE, tell your doctor about all your medical conditions, including if you:
- are allergic to pork (pig) products.
- have a history of blockage of your intestines, or scarring or thickening of your bowel wall (fibrosing colonopathy)
- have gout, kidney disease, or high blood uric acid (hyperuricemia)
- have trouble swallowing capsules
- have any other medical condition
- are pregnant or plan to become pregnant. It is not known if PANCREAZE will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if PANCREAZE passes into your breast milk. You and your doctor should decide if you will take PANCREAZE or breastfeed.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take PANCREAZE?
Take PANCREAZE exactly as your doctor tells you.
- Do not take more capsules in a day than the number your doctor tells you to take (total daily dose).
- Always take PANCREAZE with a meal or snack and plenty of fluid. If you eat a lot of meals or snacks in a day, be careful not to go over your total daily dose.
- Your doctor may change your dose based on the amount of fatty foods you eat or based on your weight.
- Do not crush or chew the PANCREAZE capsules or their contents, and do not hold the capsule or contents in your mouth. Crushing, chewing or holding the PANCREAZE capsules in your mouth may cause irritation in your mouth or change the way PANCREAZE works in your body.
Giving PANCREAZE to infants (children up to 12 months):
- Give PANCREAZE right before each feeding of formula or breast milk.
- Do not mix PANCREAZE capsule contents directly into formula or breast milk.
- Open the capsules and sprinkle the contents directly into your infant's mouth or mix the contents in a small amount of soft food such as applesauce. These foods should be the kind found in baby food jars that you buy at the store, or other food recommended by your doctor.
- If you sprinkle the PANCREAZE on food, give the PANCREAZE and food mixture to your child right away. Do not store PANCREAZE that is mixed with food.
- Give your child enough liquid to completely swallow the PANCREAZE contents or the PANCREAZE and food mixture.
- Look into your child's mouth to make sure that all of the medicine has been swallowed.
Giving PANCREAZE to children and adults
- Swallow PANCREAZE capsules whole and take them with enough liquid to swallow them right away.
- If you have trouble swallowing capsules, open the capsules and sprinkle the contents on a small amount of acidic food such as applesauce. Ask your doctor about other foods you can mix with PANCREAZE.
- If you sprinkle PANCREAZE on food, swallow it right after you mix it and drink plenty of water or juice to make sure the medicine is swallowed completely. Do not store PANCREAZE that is mixed with food.
- If you forget to take PANCREAZE, call your healthcare provider or wait until your next meal and take your usual number of capsules. Take your next dose at your usual time. Do not make up for missed doses.
What are the possible side effects of PANCREAZE?
PANCREAZE may cause serious side effects, including:
- See " What is the most important information I should know about PANCREAZE?"
- Irritation of the inside of your mouth. This can happen if PANCREAZE is not swallowed completely.
- Increase in blood uric acid levels. This may cause worsening of swollen, painful joints (gout) caused by an increase in your blood uric acid levels
- Allergic reactions including trouble with breathing, skin rashes, or swollen lips.
- Call your doctor right away if you have any of these symptoms.
The most common side effects of PANCREAZE include:
- Pain in your stomach (abdominal area)
- Gas
Other possible side effects of PANCREAZE:
PANCREAZE and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs.
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of PANCREAZE. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to VIVUS, Inc. at 1-888-998-4887.
How should I store PANCREAZE?
- Store PANCREAZE at room temperature below 77°F (25°C). Avoid heat.
- Keep PANCREAZE in a dry place and in the original container.
- After opening the bottle, keep it closed tightly between uses.
- The PANCREAZE 2,600 USP units of lipase, the PANCREAZE 4,200 USP units of lipase and PANCREAZE 21,000 USP units of lipase bottles contain a desiccant canister. Do not eat or throw away the desiccant canister in your medicine bottle. This canister will protect your medicine from moisture.
Keep PANCREAZE and all medicines out of the reach of children.
General information about PANCREAZE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PANCREAZE for a condition for which it was not prescribed. Do not give PANCREAZE to other people to take, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about PANCREAZE. If you would like more information, talk to your doctor. You can ask your pharmacist or doctor for information about PANCREAZE that is written for healthcare professionals.
For more information go to www.pancreaze.net or call 1-888-998-4887.
What are the ingredients in PANCREAZE?
Active Ingredient: lipase, protease, amylase
Inactive ingredients in PANCREAZE 2,600 USP units of lipase: colloidal silicon dioxide, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, microcrystalline cellulose, montan glycol wax, simethicone emulsion, talc and triethyl citrate. The capsule shell contains hypromellose, titanium dioxide, iron oxide, and imprint ink contains iron oxide, shellac, ammonium hydroxide, propylene glycol, potassium hydroxide.
Inactive ingredients in other strengths: cellulose, colloidal anhydrous silica, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, montan glycol wax, simethicone emulsion, talc and triethyl citrate. The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, sorbitan monolaurate, and gelatin capsule imprint ink. PANCREAZE 4,200, 10,500, and 16,800 USP units of lipase also contain iron oxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Product of Germany
Finished Product Manufactured by:
Nordmark Arzneimittel GmbH & Co. KG
25436 Uetersen, Germany.
Manufactured for:
VIVUS, Inc.
900 E. Hamilton Ave., Suite 550
Campbell, CA 95008, USA
©VIVUS, Inc. 2010
PH-10-001-00
-
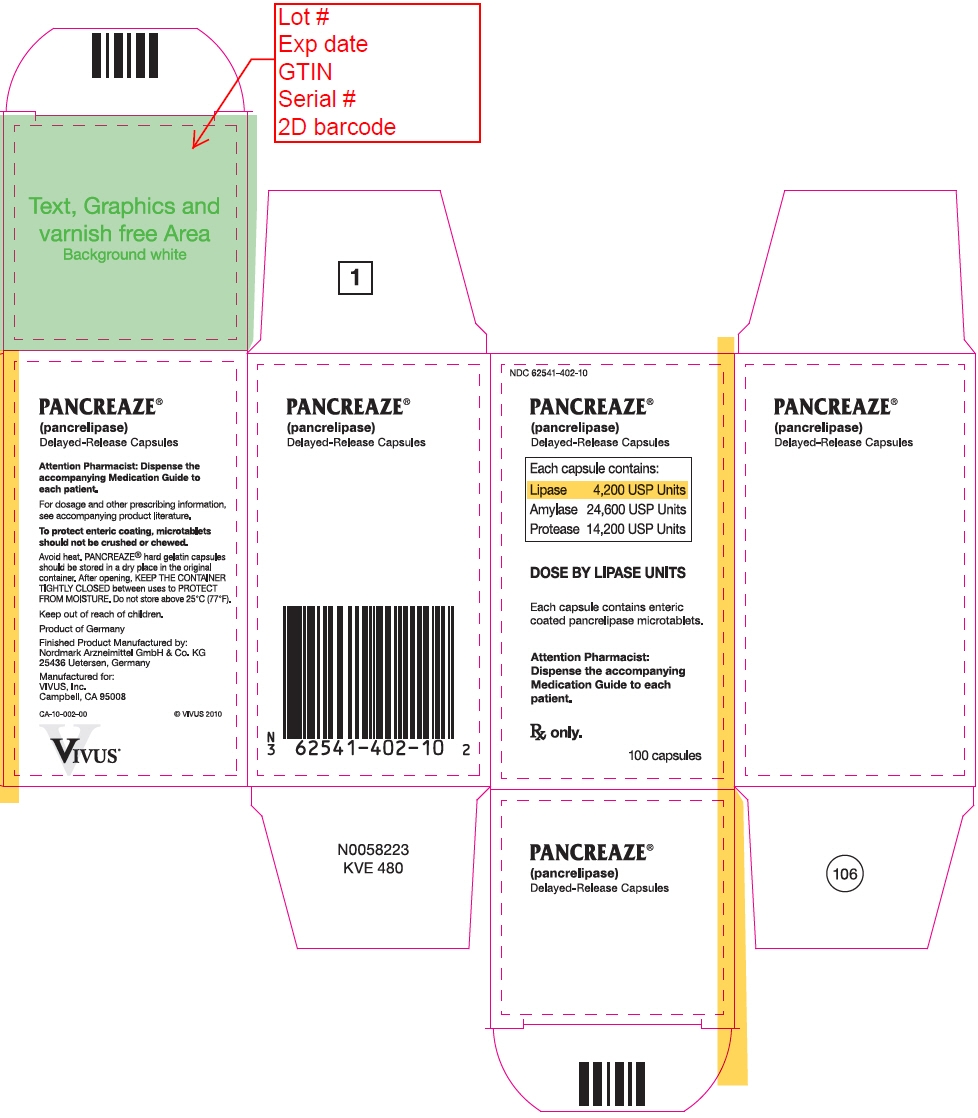
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Carton - NDC: 62541-402-10
NDC: 62541-402-10
PANCREAZE ®
(pancrelipase)
Delayed-Release CapsulesEach capsule contains:
Lipase 4,200 USP Units
Amylase 24,600 USP Units
Protease 14,200 USP UnitsDOSE BY LIPASE UNITS
Each capsule contains enteric
coated pancrelipase microtablets.Attention Pharmacist:
Dispense the accompanying
Medication Guide to each
patient.Rx only.
100 capsules
-
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Carton - NDC: 62541-403-10
NDC: 62541-403-10
PANCREAZE ®
(pancrelipase)
Delayed-Release CapsulesEach capsule contains:
Lipase 10,500 USP Units
Amylase 61,500 USP Units
Protease 35,500 USP UnitsDOSE BY LIPASE UNITS
Each capsule contains enteric coated
pancrelipase microtablets.Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient.Rx only.
100 capsules

-
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Carton - NDC: 62541-404-10
NDC: 62541-404-10
PANCREAZE ®
(pancrelipase)
Delayed-Release CapsulesEach capsule contains:
Lipase 16,800 USP Units
Amylase 98,400 USP Units
Protease 56,800 USP UnitsDOSE BY LIPASE UNITS
Each capsule contains enteric coated
pancrelipase microtablets.Attention Pharmacist:
Dispense the accompanying
Medication Guide to each
patient.Rx only.
100 capsules

-
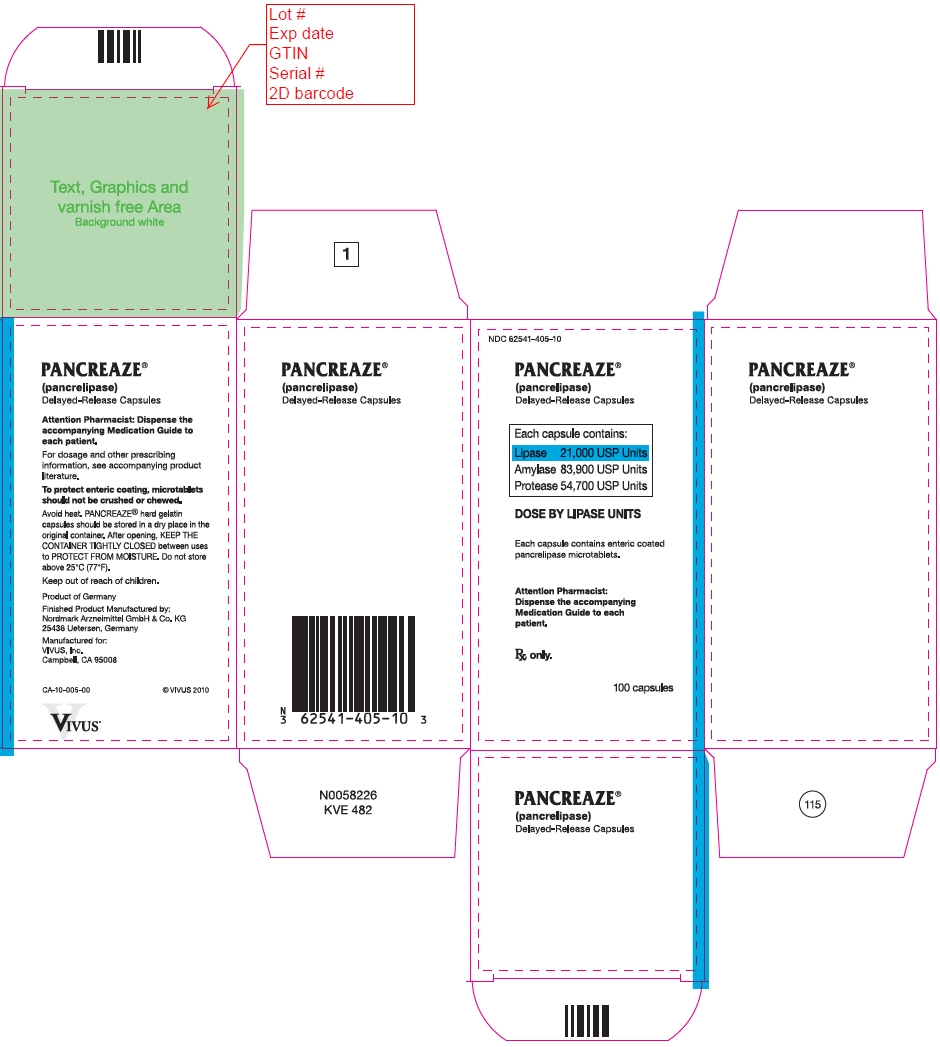
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Carton - NDC: 62541-405-10
NDC: 62541-405-10
PANCREAZE ®
(pancrelipase)
Delayed-Release CapsulesEach capsule contains:
Lipase 21,000 USP Units
Amylase 83,900 USP Units
Protease 54,700 USP UnitsDOSE BY LIPASE UNITS
Each capsule contains enteric coated
pancrelipase microtablets.Attention Pharmacist:
Dispense the accompanying
Medication Guide to each
patient.Rx only.
100 capsules
-
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Carton - NDC: 62541-401-10
NDC: 62541-401-10
PANCREAZE ®
(pancrelipase)
Delayed-Release CapsulesEach capsule contains:
Lipase 2,600 USP Units
Amylase 10,850 USP Units
Protease 6,200 USP UnitsDOSE BY LIPASE UNITS
Each capsule contains enteric
coated pancrelipase microtablets.Attention Pharmacist:
Dispense the accompanying
Medication Guide to each
patient.Rx only.
100 capsules

-
INGREDIENTS AND APPEARANCE
PANCREAZE
pancrelipase lipase, pancrelipase amylase, and pancrelipase protease capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62541-402 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 4200 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 24600 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 14200 [USP'U] Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color yellow (Opaque) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code VIVUS;MT;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62541-402-10 1 in 1 CARTON 04/12/2010 1 100 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022523 04/12/2010 PANCREAZE
pancrelipase lipase, pancrelipase amylase, and pancrelipase protease capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62541-403 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 10500 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 61500 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 35500 [USP'U] Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color pink (Opaque) Score no score Shape CAPSULE Size 20mm Flavor Imprint Code VIVUS;MT;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62541-403-10 1 in 1 CARTON 04/12/2010 1 100 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022523 04/12/2010 PANCREAZE
pancrelipase lipase, pancrelipase amylase, and pancrelipase protease capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62541-404 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 16800 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 98400 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 56800 [USP'U] Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color orange (Salmon Opaque) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code VIVUS;MT;16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62541-404-10 1 in 1 CARTON 04/12/2010 1 100 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022523 04/12/2010 PANCREAZE
pancrelipase lipase, pancrelipase amylase, and pancrelipase protease capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62541-405 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 21000 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 83900 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 54700 [USP'U] Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color white (Opaque) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code VIVUS;MT;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62541-405-10 1 in 1 CARTON 04/12/2010 1 100 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022523 04/12/2010 PANCREAZE
pancrelipase lipase, pancrelipase amylase, and pancrelipase protease capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62541-401 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 2600 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 10850 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 6200 [USP'U] Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) PROPYLENE GLYCOL DIPROPIONATE (UNII: 4DZ729T8XW) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color orange (light orange opaque) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code VIVUS;MT;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62541-401-10 1 in 1 CARTON 08/01/2016 1 100 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022523 08/01/2016 Labeler - VIVUS, Inc. (782772263) Establishment Name Address ID/FEI Business Operations Nordmark Arzneimittel GmbH & Co. KG 314826350 analysis(62541-402, 62541-403, 62541-404, 62541-405, 62541-401) , api manufacture(62541-401, 62541-402, 62541-403, 62541-404, 62541-405) , manufacture(62541-401, 62541-402, 62541-403, 62541-404, 62541-405)
Trademark Results [PANCREAZE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PANCREAZE 77817201 3887562 Live/Registered |
VIVUS, INC. 2009-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.