YUTREPIA- treprostinil capsule
Yutrepia by
Drug Labeling and Warnings
Yutrepia by is a Prescription medication manufactured, distributed, or labeled by Liquidia Technologies, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use YUTREPIA™ safely and effectively. See full prescribing information for YUTREPIA™.
YUTREPIA™ (treprostinil) inhalation powder, for oral inhalation
Initial U.S. Approval: 2002INDICATIONS AND USAGE
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%). (1.1)
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%). (1.2)
DOSAGE AND ADMINISTRATION
- For oral inhalation only. Do not swallow YUTREPIA capsules. Use only with the provided inhaler (2)
- YUTREPIA should be administered 3 to 5 times per day. The contents of each capsule can be inhaled in 2 breaths. (2.1)
- See Dosage and Administration for full instructions on dosing of patients who are treprostinil-naïve or transitioning from treprostinil inhalation solution to YUTREPIA (2.1)
DOSAGE FORMS AND STRENGTHS
YUTREPIA inhalation powder contained in capsule is available in 4 strengths: 26.5 mcg, 53 mcg, 79.5 mcg, 106 mcg (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Treprostinil may cause symptomatic hypotension. (5.1)
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding. (5.2)
- Dosage adjustments may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn. (5.3, 7.1)
- May cause bronchospasm: Patients with a history of hyperreactive airway disease may be more sensitive. (5.4)
ADVERSE REACTIONS
Most common adverse reactions with YUTREPIA (³10%) are cough, headache, throat irritation, and dizziness. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Liquidia Technologies, Inc. at 1-888-393-LQDA (5732) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
1.2 Pulmonary Hypertension Associated with ILD
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage In Adults
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Symptomatic Hypotension
5.2 Risk of Bleeding
5.3 Effect of Other Drugs on Treprostinil
5.4 Bronchospasm
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Adverse Reactions Identified in Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Cytochrome P450 Inhibitors and Inducers
7.2 Effect of Other Drugs on Treprostinil
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Insufficiency
8.7 Patients with Renal Insufficiency
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension (WHO Group 1)
14.2 Pulmonary Hypertension Associated with ILD (WHO Group 3)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
YUTREPIA is indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
The effects diminish over the minimum recommended dosing interval of 4 hours; treatment timing can be adjusted for planned activities.
While there are long-term data on use of treprostinil by other routes of administration, nearly all controlled clinical experience with inhaled treprostinil has been on a background of bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase type 5 inhibitor). The controlled clinical experience was limited to 12 weeks in duration [see Clinical Studies (14)].
1.2 Pulmonary Hypertension Associated with ILD
YUTREPIA is indicated for the treatment of pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%) [see Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage In Adults
YUTREPIA capsules are for oral inhalation only and should be used only with the supplied inhaler. Do not swallow YUTREPIA capsules.
YUTREPIA Dosing in treprostinil-naïve patients:
In patients naïve to treprostinil, therapy should begin with 26.5 mcg 3 to 5 times per day, in 2 breaths based on patient response.
Dosing in patients transitioning from treprostinil inhalation solution (Tyvaso):
Patients transitioning from treprostinil inhalation solution (Tyvaso), can begin YUTREPIA therapy 3 to 5 times per day, in 2 breaths, using the doses specified below (Table 1):
Table 1: YUTREPIA Dosing in Patients Transitioning from Treprostinil Inhalation Solution *Each breath of Tyvaso delivers approximately 6 mcg of treprostinil. Current Tyvaso Dose*
YUTREPIA Dose
(Number of Breaths)
mcg
5 or less breaths
26.5 mcg
6 to 8 breaths
53 mcg
9 to 11 breaths
79.5 mcg
12 to 14 breaths
106 mcg
15 to 17 breaths
132.5 mcg
18 or more breaths
159 mcg
In treprostinil-naïve patients and those transitioning from treprostinil inhalation solution, dose increases of 26.5 mcg per dose each week may be implemented, as tolerated. The target maintenance dosage is 79.5 mcg to 106 mcg, 4 times daily.
Doses above 848 mcg per day have not been studied in patients with PAH.
If a scheduled dose is missed, resume therapy as soon as possible at the usual dose.
-
3 DOSAGE FORMS AND STRENGTHS
YUTREPIA inhalation powder contained in capsule available in 4 strengths:
- 26.5 mcg: opaque yellow cap and clear body capsule with “LIQUIDIA 26.5” in black radial imprint on capsule cap.
- 53 mcg: opaque green cap and clear body capsule with “LIQUIDIA 53” in white radial imprint on capsule cap.
- 79.5 mcg: opaque blue cap and clear body capsule with “LIQUIDIA 79.5” in white radial imprint on capsule cap.
- 106 mcg: opaque purple cap and clear body capsule with “LIQUIDIA 106” in white radial imprint on capsule cap.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Symptomatic Hypotension
Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
5.3 Effect of Other Drugs on Treprostinil
Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
5.4 Bronchospasm
Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
-
6 ADVERSE REACTIONS
The following potential adverse reactions are described in Warnings and Precautions (5):
- - Decrease in systemic blood pressure [see Warnings and Precautions (5.1)].
- - Bleeding [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. Table 2 lists the adverse reactions that occurred at a rate of at least 4% of the overall INSPIRE safety population. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
Table 2: Adverse Reactions Occurring in ≥ 4% of Patients in the INSPIRE Study *Transition: Patients were on stable doses of treprostinil inhalation solution for at least 3 months prior to enrollment in the study and transitioned to treatment with YUTREPIA.
†Add-on: Patients were prostacyclin-naïve and were taking no more than 2 approved oral PAH therapies for at least 3 months at time of enrollment and addition of treatment with YUTREPIA.Adverse Reaction
Transition*
N=55Add-On†
N=66n (%)
n (%)
Cough
15 (27)
36 (55)
Headache
14 (25)
18 (27)
Throat Irritation
5 (9)
14 (21)
Dizziness
6 (11)
7 (11)
Diarrhea
3 (6)
8 (12)
Chest Discomfort
5 (9)
5 (8)
Nausea
4 (7)
5 (8)
Dyspnea
3 (6)
3 (5)
Flushing
1 (2)
5 (8)
Oropharyngeal Pain
1 (2)
4 (6)
6.2 Adverse Reactions Identified in Post-Marketing Experience
The following adverse reaction has been identified during the post-approval use of treprostinil inhalation solution. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure:
- Angioedema
-
7 DRUG INTERACTIONS
7.1 Effect of Cytochrome P450 Inhibitors and Inducers
In vitro studies of human hepatic microsomes showed that treprostinil does not inhibit cytochrome P450 (CYP) isoenzymes CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A.
Additionally, treprostinil does not induce cytochrome P450 isoenzymes CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A.
Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor gemfibrozil increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer rifampin decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8 [see Warnings and Precautions (5.3)].
7.2 Effect of Other Drugs on Treprostinil
Drug interaction studies have been carried out with treprostinil (oral or subcutaneous) co-administered with acetaminophen (4 g/day), warfarin (25 mg/day), and fluconazole (200 mg/day), respectively, in healthy volunteers. These studies did not show a clinically significant effect on the pharmacokinetics of treprostinil. Treprostinil does not affect the pharmacokinetics or pharmacodynamics of warfarin. The pharmacokinetics of R- and S- warfarin and the INR in healthy subjects given a single 25 mg dose of warfarin were unaffected by continuous subcutaneous infusion of treprostinil at an infusion rate of 10 ng/kg/min.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, there are risks to the mother and the fetus associated with pulmonary arterial hypertension (see Clinical Considerations). In animal studies, no adverse reproductive and developmental effects were seen for treprostinil at 39 and 3145 times the human exposure when based on Cmax and AUC, respectively, following a single YUTREPIA dose of 79.5 mcg [see Clinical Pharmacology (12.3)].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo-fetal risk
Pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality.
Data
Animal reproduction studies have been conducted with treprostinil via continuous subcutaneous administration and with treprostinil diolamine administered orally. In studies with orally administered treprostinil diolamine, no adverse effect doses for fetal viability/growth, fetal development (teratogenicity), and postnatal development were determined in rats. In pregnant rats, no evidence of harm to the fetus was observed following oral administration of treprostinil diolamine at the highest dose tested (20 mg/kg/day), which represents about 154 and 1479 times the human exposure, when based on Cmax and AUC, respectively, following a single YUTREPIA dose of 79.5 mcg. In pregnant rabbits, external fetal and soft tissue malformations and fetal skeletal malformation occurred. The dose at which no adverse effects were seen (0.5 mg/kg/day) represents about 9 and 145 times the human exposure, when based on Cmax and AUC, respectively, following a single YUTREPIA dose of 79.5 mcg. No treprostinil treatment-related effects on labor and delivery were seen in animal studies. Animal reproduction studies are not always predictive of human response.
8.5 Geriatric Use
Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open-label INSPIRE study in PAH patients included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
8.6 Patients with Hepatic Insufficiency
Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects with mild-to-moderate hepatic insufficiency. Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency [see Clinical Pharmacology (12.3)].
8.7 Patients with Renal Insufficiency
No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
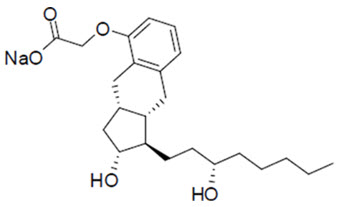
YUTREPIA contains treprostinil sodium, a prostacyclin mimetic. The chemical name for tresprostinil sodium is 2-{[(1R,2R,3aS,9aS)-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H,2H,3H,3aH,4H,9H,9aH-cyclopenta[b]naphthalen-5-yl]oxy}acetic acid, sodium salt with the structural formula:
Treprostinil sodium has a molecular formula of C23H33O5Na and a molecular weight of 412.49 daltons equivalent to 390.5 daltons of Treprostinil.
YUTREPIA inhalation powder contained in a capsule is intended for oral inhalation. The capsule contains white to off-white powder of treprostinil sodium and the inactive ingredients L-leucine, polysorbate 80, sodium chloride, sodium citrate, and trehalose. Each 5 mg of YUTREPIA inhalation powder contains 26.5 mcg of treprostinil, where 26.5 mcg of treprostinil is equivalent to 28 mcg of treprostinil sodium.
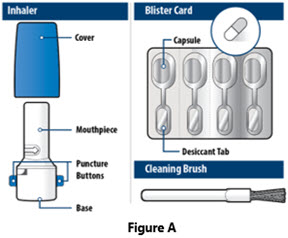
The accompanying inhalation device for delivery of YUTREPIA inhalation powder is a disposable plastic device used to inhale the dry powder contained in the HPMC capsule.
The amount of drug delivered to the lungs will vary depending on patient factors such as inspiratory flow and peak inspiratory flow through the inhalation device, which may vary from patient to patient.
Under standardized in vitro testing, the inhalation device delivers the following amounts of treprostinil for each of the YUTREPIA inhalation powder capsule strengths:
YUTREPIA Inhalation Powder Delivered Dose
a Amount of treprostinil delivered from the device mouthpiece under an in vitro flow rate of 99 L/min with a collection time of 1.2 seconds (2 L total volume). Capsule Strength
(treprostinil)Dose Delivered a
26.5 mcg
15.1 mcg
53 mcg
36.0 mcg
79.5 mcg
56.6 mcg
106 mcg
75.7 mcg
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation.
12.2 Pharmacodynamics
In animals, the vasodilatory effects reduce right and left ventricular afterload and increase cardiac output and stroke volume. Other studies have shown that treprostinil causes a dose-related negative inotropic and lusitropic effect. No major effects on cardiac conduction have been observed. Treprostinil produces vasodilation and tachycardia.
Cardiac Electrophysiology
In a clinical trial of 240 healthy volunteers, single doses of treprostinil inhalation solution 54 mcg (the target maintenance dose per session) and 84 mcg (supratherapeutic inhalation dose) prolonged the corrected QTc interval by approximately 10 ms. The QTc effect dissipated rapidly as the concentration of treprostinil decreased.
12.3 Pharmacokinetics
Absorption
In healthy volunteer studies, the systemic exposure (AUC and Cmax) post-inhalation was shown to be proportional to the YUTREPIA doses administered (25 mcg – 150 mcg). The treprostinil mean Cmax, mean AUCinf and median Tmax following a single inhaled target maintenance dose of 79.5 mcg YUTREPIA were 1.48 ng/mL, 1.04 hr.ng/mL and 0.13 hr, respectively.
Distribution
In vitro treprostinil is 91% bound to human plasma proteins over the 330-10,000 ng/mL concentration range.
Metabolism and Excretion
Of subcutaneously administered treprostinil, only 4% is excreted unchanged in urine. Treprostinil is substantially metabolized by the liver, primarily by CYP2C8. Metabolites are excreted in urine (79%) and feces (13%) over 10 days. Five apparently inactive metabolites were detected in the urine, each accounting for 10‑15% of the dose administered. Four of the metabolites are products of oxidation of the 3-hydroxyloctyl side chain and one is a glucuroconjugated derivative (treprostinil glucuronide).
Elimination
Following inhaled administration of YUTREPIA, disposition and elimination is monophasic with a half-life of approximately 30 minutes.
Specific Populations
Hepatic Insufficiency
Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects presenting with mild-to-moderate hepatic insufficiency. Treprostinil has not been studied in patients with severe hepatic insufficiency [see Use in Specific Populations (8.6)].
Renal Insufficiency
In patients with severe renal impairment requiring dialysis (n=8), administration of a single 1 mg dose of orally administered treprostinil pre-and post-dialysis resulted in AUC0-inf that was not significantly altered compared to healthy subjects [see Use in Specific Populations (8.7)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A two-year rat carcinogenicity study was performed with treprostinil inhalation solution at target treprostinil doses of 5.26, 10.6, and 34.1 mcg/kg/day. There was no evidence for carcinogenic potential associated with treprostinil inhalation in rats at systemic exposure levels up to 35 times following a single YUTREPIA dose of 79.5 mcg [see Clinical Pharmacology (12.3)]. In vitro and in vivo genetic toxicology studies did not demonstrate any mutagenic or clastogenic effects of treprostinil. Treprostinil sodium did not affect fertility or mating performance of male or female rats given continuous subcutaneous (sc) infusions at rates of up to 450 ng treprostinil/kg/min. In this study, males were dosed from 10 weeks prior to mating and through the 2-week mating period. Females were dosed from 2 weeks prior to mating until gestational day 6.
Oral administration of treprostinil diolamine to Tg.rasH2 mice at 0, 5, 10 and 20 mg/kg/day in males and 0, 3, 7.5 and 15 mg/kg/day in females daily for 26 weeks did not significantly increase the incidence of tumors.
Treprostinil diolamine was tested in vivo in a rat micronucleus assay and did not induce an increased incidence of micronucleated polychromatic erythrocytes.
13.2 Animal Toxicology and/or Pharmacology
In a 2-year rat study with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day, there were more deaths (11) in the mid- and high-dose treprostinil groups during the first 9 weeks of the study, compared to 1 in control groups. At the high-dose level, males showed a higher incidence of inflammation in teeth and preputial gland, and females showed high incidences of inflammation and urothelial hyperplasia in the urinary bladder. The exposures in rats at mid- and high-dose levels were about 15 and 35 times, respectively, the clinical exposure following a single YUTREPIA dose of 79.5 mcg [see Clinical Pharmacology (12.3)].
-
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension (WHO Group 1)
TRIUMPH I was a 12-week, randomized, double-blind, placebo-controlled multi-center study of patients with PAH. The study population included 235 clinically stable patients with pulmonary arterial hypertension (WHO Group 1), nearly all with NYHA Class III (98%) symptoms who were receiving either bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase-5 inhibitor) for at least three months prior to study initiation. Concomitant therapy also could have included anticoagulants, other vasodilators (e.g., calcium channel blockers), diuretics, oxygen, and digitalis, but not a prostacyclin. These patients were administered either placebo or treprostinil inhalation solution in four daily treatment sessions with a target dose of 9 breaths (equivalent to 79.5 mcg YUTREPIA) per session over the course of the 12-week study. Patients were predominantly female (82%), had the origin of PAH as idiopathic/heritable (56%), secondary to connective tissue diseases (33%) or secondary to HIV or previous use of anorexigens (12%); bosentan was the concomitant oral medication in 70% of those enrolled, sildenafil in 30%.
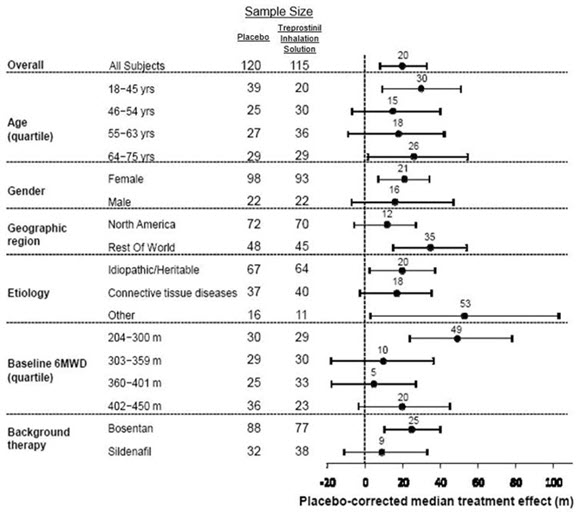
The primary efficacy endpoint of the trial was the change in six-minute walk distance (6MWD) relative to baseline at 12 weeks. 6MWD was measured at peak exposure (between 10 and 60 minutes after dosing), and 3‑5 hours after bosentan or 0.5-2 hours after sildenafil. Patients receiving treprostinil inhalation solution had a placebo-corrected median change from baseline in peak 6MWD of 20 meters at Week 12 (p<0.001).
The distribution of these 6MWD changes from baseline at Week 12 were plotted across the range of observed values (Figure 1). 6MWD measured at trough exposure (defined as measurement of 6MWD at least 4 hours after dosing) improved by 14 meters. There were no placebo-controlled 6MWD assessments made after 12 weeks.
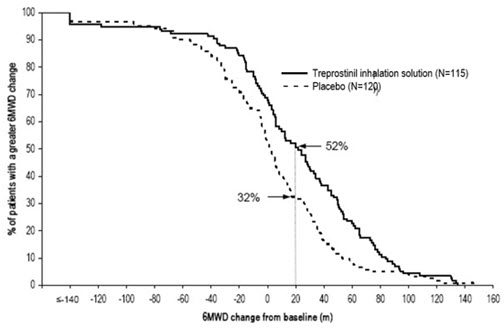
Figure 1. Distributions of 6MWD Changes from Baseline at Week 12 during Peak Plasma Concentration of Treprostinil Inhalation Solution
The placebo-corrected median treatment effect on 6MWD was estimated (using the Hodges-Lehmann estimator) within various subpopulations defined by age quartile, gender, geographic region of the study site, disease etiology, baseline 6MWD quartile, and type of background therapy (Figure 2).
14.2 Pulmonary Hypertension Associated with ILD (WHO Group 3)
INCREASE was a 16-week, randomized, double-blind, placebo-controlled, multicenter study that enrolled 326 patients with PH-ILD. Enrolled study patients predominately had etiologies of idiopathic interstitial pneumonia (45%) inclusive of idiopathic pulmonary fibrosis, combined pulmonary fibrosis and emphysema (25%), and WHO Group 3 connective tissue disease (22%). The mean baseline 6MWD was 260 meters.
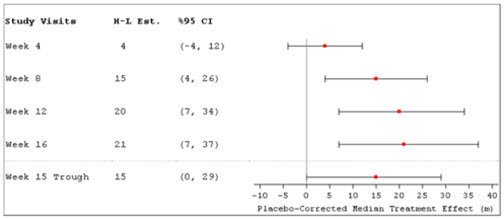
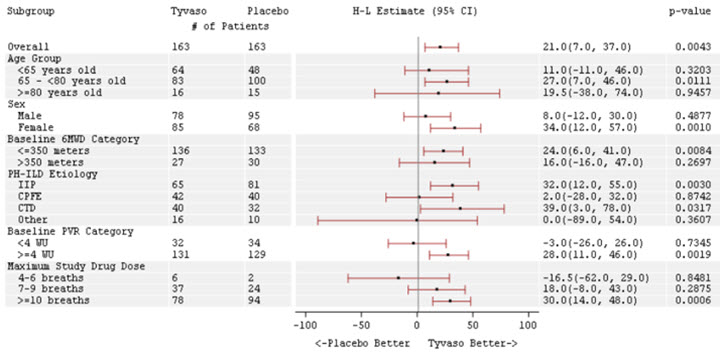
Patients in the INCREASE study were randomized (1:1) to either placebo or treprostinil inhalation solution in four daily treatment sessions with a target dose of 9 breaths (equivalent to 79.5 mcg YUTREPIA) per session and a maximum dose of 12 breaths (equivalent to 106 mcg YUTREPIA) per session over the course of the 16-week study. Approximately 75% of patients randomized to treprostinil inhalation solution titrated up to a dose of 9 breaths, 4 times daily or greater, with 48% of patients randomized to treprostinil inhalation solution reaching a dose of 12 breaths, 4 times daily during the study. The primary efficacy endpoint was the change in 6MWD measured at peak exposure (between 10 and 60 minutes after dosing) from baseline to Week 16. Patients receiving treprostinil inhalation solution had a placebo-corrected median change from baseline in peak 6MWD of 21 meters at Week 16 (p=0.004) using Hodges-Lehmann estimate (Figure 3).
The treatment effect on 6MWD at Week 16 was consistent for various subgroups, including etiology of PH-ILD, disease severity, age, sex, baseline hemodynamics, and dose (Figure 4).
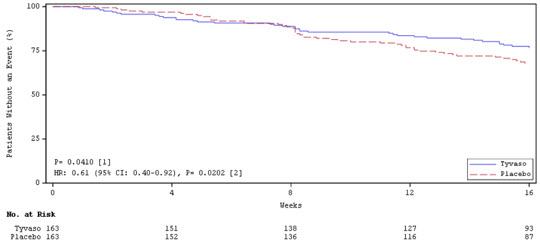
Time to clinical worsening in the INCREASE study was defined as the time of randomization until 1 of the following criteria were met: hospitalization due to a cardiopulmonary indication, decrease in 6MWD >15% from baseline directly related to PH-ILD at 2 consecutive visits and at least 24 hours apart, death (all causes), or lung transplantation. Treatment with treprostinil inhalation solution in patients with PH-ILD resulted in numerically fewer hospitalizations. The numbers of reported deaths were the same for both treatment groups (Table 3). Overall, treatment with treprostinil inhalation solution demonstrated a statistically significant increase in the time to first clinical worsening event (log-rank test p=0.041; Figure 5), and a 39% overall reduction in the risk of a clinical worsening event (HR=0.61 [95% CI; 0.40, 0.92]; Figure 5).
Table 3: Clinical Worsening Events (PH-ILD) Tyvaso
n=163
n (%)Placebo
n=163
n (%)HR (95% CI)
Clinical worsening
37 (22.7%)
54 (33.1%)
0.61 (0.40, 0.92)
First contributing event
Hospitalization due to a cardiopulmonary indication
18 (11.0%)
24 (14.7%)
Decrease in 6MWD > 15% from baseline directly related to PH-ILD
13 (8.0%)
26 (16.0%)
Death (all causes)
4 (2.5%)
4 (2.5%)
Lung transplantation
2 (1.2%)
0
Tyvaso
n=163
n (%)Placebo
n=163
n (%)HR (95% CI)
First of each event
Hospitalization due to a cardiopulmonary indication
21 (12.9)
30 (18.4%)
Decrease in 6MWD > 15% from baseline directly related to PH-ILD
16 (9.8%)
31 (19.0%)
Death (all causes)
8 (4.9%)
10 (6.1%)
Lung transplantation
2 (1.2%)
1 (0.6%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
YUTREPIA is supplied in a carton consisting of 1 capsule based, dry powder inhaler (referred to as “inhaler”), 28 capsules (7 foil blister cards of 4 capsules each), and 7 single-use cleaning brushes. The individual capsule well is connected by an air channel to a separate blister well containing a desiccant strip. Descriptions of YUTREPIA carton by capsule strength are provided in Table 4 below:
Table 4: YUTREPIA Carton Contents by Capsule Strength Capsule Strength
(mcg treprostinil)Capsule Description NDC Number 26.5
Opaque yellow cap, clear body, imprinted with “LIQUIDIA 26.5” in black ink radially on cap
72964-011-01
53
Opaque green cap, clear body, imprinted with “LIQUIDIA 53” in white ink radially on cap
72964-012-01
79.5
Opaque blue cap, clear body, imprinted with “LIQUIDIA 79.5” in white ink radially on cap
72964-013-01
106
Opaque purple cap, clear body, imprinted with “LIQUIDIA 106” in white ink radially on cap
72964-014-01
YUTREPIA inhalation powder capsules should only be delivered using the capsule-based inhaler. The off-white plastic inhaler consists of a blue protective cap marked with YUTREPIA and a base with a mouthpiece, capsule chamber, and two blue push buttons. Discard the inhaler device after 7 days of use or 56 actuations, whichever comes first.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
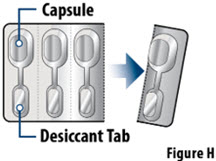
Capsules should remain in the blister to protect them from moisture and light, and each capsule should be removed only when ready to administer a dose.
Keep out of the reach of children.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Train patients in the administration process for YUTREPIA, including dosing, inhaler preparation, administration, cleaning, and maintenance, according to the instructions for use [see Instructions for Use].
To avoid potential interruptions in drug delivery because of equipment malfunction, patients should have access to a back-up.
If a scheduled dose is missed, resume therapy as soon as possible at the usual dose.
©Copyright 2025 Liquidia Technologies, Inc. All rights reserved.
Distributed by: Liquidia Technologies, Inc. Morrisville, NC 27560
-
Instructions for Use
YUTREPIATM (you-TREP-ee-uh)
(treprostinil)
inhalation powder, for oral inhalationThis Instructions for Use contains information on how to inhale YUTREPIATM. Read these Instructions for Use before you start using YUTREPIA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Your healthcare provider should show you or your caregiver how to use YUTREPIA the right way before you use it for the first time.
Important information you need to know before inhaling YUTREPIA inhalation powder:
- Do not swallow YUTREPIA capsules. YUTREPIA is for inhalation only.
- Use YUTREPIA as prescribed by your healthcare provider.
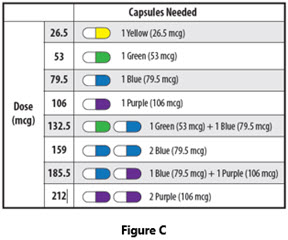
- YUTREPIA capsules come in 4 strengths: 26.5 mcg, 53 mcg, 79.5 mcg, and 106 mcg.
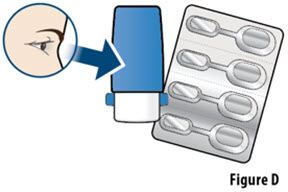
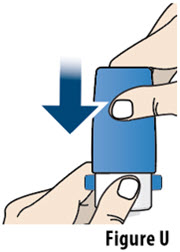
- If your prescribed dose is more than 106 mcg, you will need to inhale 2 YUTREPIA capsules. See Figure C: Dosing Chart to help you identify the 2 capsules needed for your prescribed dose. Only use the capsule combinations in the Dosing Chart when your prescribed dose is more than 106 mcg.
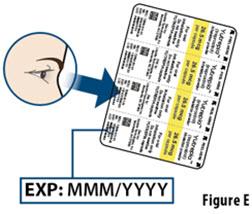
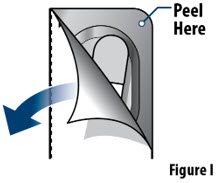
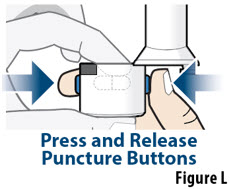
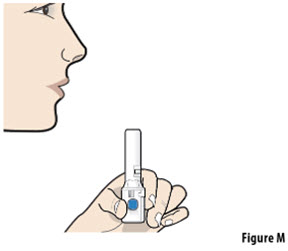
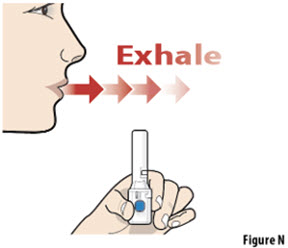
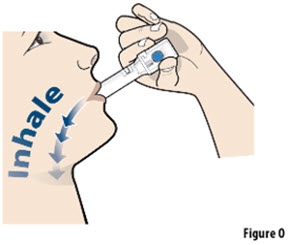
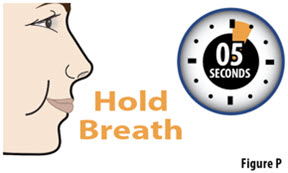
- The capsule must be inhaled within 5 minutes of opening the blister card or the full dose may not be administered. Read through this instruction sheet prior to the first use of this product.
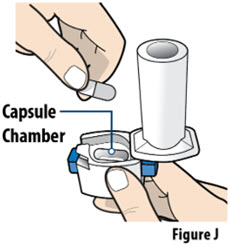
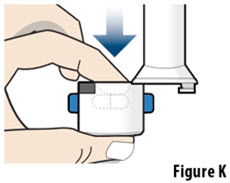
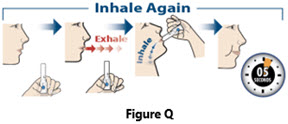
- Always inhale each capsule 2 times to make sure you get your full dose of YUTREPIA.
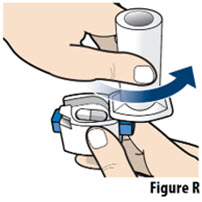
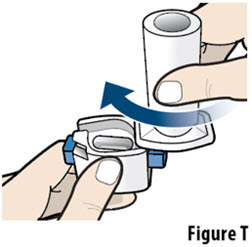
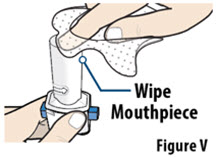
- Do not wash the inhaler. Keep the inhaler dry.
- Wash and dry your hands before using YUTREPIA.
- If the contents of the capsule comes in contact with your skin or eyes, rinse the area immediately with water.
- YUTREPIA capsules should remain in the blister card(s) and each capsule should be removed only when ready to deliver a dose.
Storing YUTREPIA
- Store YUTREPIA carton in a clean, dry place at room temperature between 68°F to 77°F (20°C to 25°C).
- Leave YUTREPIA capsules in blister card to protect from moisture and light.
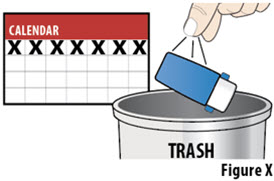
- Throw away the inhaler after 7 days of use or 56 capsules whichever comes first.
- Keep YUTREPIA and all medicines out of the reach of children.
©Copyright 2025 Liquidia Technologies, Inc. All rights reserved.
Distributed by: Liquidia Technologies, Inc. Morrisville, NC 27560
For more information call 1-888-393-LQDA (5732) or go to www.YUTREPIA.comThis Instructions for Use has been approved by the U.S. Food and Drug Administration
Issued: June 2025
-
PRINCIPAL DISPLAY PANEL – 26.5 mcg Carton
NDC: 72964-011-01
Rx Only26.5 mcg
per capsuleYutrepia™
(treprostinil) inhalation powderFor oral inhalation only
DO NOT SWALLOW YUTREPIA CAPSULESContents:
- 28 Capsules (7 Foil blister cards of 4 capsules each)
- 1 Dry powder inhaler
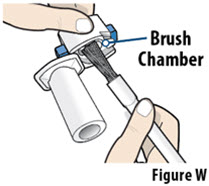
- 7 Single-use cleaning brushes
- Instructions for Use
Liquidia™
NDC: 72964-011-01
Rx Only26.5 mcg
per capsuleYutrepia™
(treprostinil)
inhalation powderRead Instructions for Use before each use.
Recommended Dosage: See prescribing information.
Do not remove capsules from blister cards until immediately before use.
Store at 68°F to 77°F (20°C to 25°C); excursions permitted to 59°F to
86°F (15°C to 30°C) [see USP Controlled Room Temperature].
Keep this and all drugs out of the reach of children.Distributed by:
Liquidia Technologies Inc.
Morrisville, NC 27560Liquidia™
-
PRINCIPAL DISPLAY PANEL – 53 mcg Carton
NDC: 72964-012-01
Rx Only53 mcg
per capsuleYutrepia™
(treprostinil) inhalation powderFor oral inhalation only
DO NOT SWALLOW YUTREPIA CAPSULESContents:
- 28 Capsules (7 Foil blister cards of 4 capsules each)
- 1 Dry powder inhaler
- 7 Single-use cleaning brushes
- Instructions for Use
Liquidia™
NDC: 72964-012-01
Rx Only53 mcg
per capsuleYutrepia™
(treprostinil)
inhalation powderRead Instructions for Use before each use.
Recommended Dosage: See prescribing information.
Do not remove capsules from blister cards until immediately before use.
Store at 68°F to 77°F (20°C to 25°C); excursions permitted to 59°F to
86°F (15°C to 30°C) [see USP Controlled Room Temperature].
Keep this and all drugs out of the reach of children.Distributed by:
Liquidia Technologies Inc.
Morrisville, NC 27560Liquidia™
-
PRINCIPAL DISPLAY PANEL – 79.5 mcg Carton
NDC: 72964-013-01
Rx Only79.5 mcg
per capsuleYutrepia™
(treprostinil) inhalation powderFor oral inhalation only
DO NOT SWALLOW YUTREPIA CAPSULESContents:
- 28 Capsules (7 Foil blister cards of 4 capsules each)
- 1 Dry powder inhaler
- 7 Single-use cleaning brushes
- Instructions for Use
Liquidia™
NDC: 72964-013-01
Rx Only79.5 mcg
per capsuleYutrepia™
(treprostinil)
inhalation powderRead Instructions for Use before each use.
Recommended Dosage: See prescribing information.
Do not remove capsules from blister cards until immediately before use.
Store at 68°F to 77°F (20°C to 25°C); excursions permitted to 59°F to
86°F (15°C to 30°C) [see USP Controlled Room Temperature].
Keep this and all drugs out of the reach of children.Distributed by:
Liquidia Technologies Inc.
Morrisville, NC 27560Liquidia™
-
PRINCIPAL DISPLAY PANEL – 106 mcg Carton
NDC: 72964-014-01
Rx Only106 mcg
per capsuleYutrepia™
(treprostinil) inhalation powderFor oral inhalation only
DO NOT SWALLOW YUTREPIA CAPSULESContents:
- 28 Capsules (7 Foil blister cards of 4 capsules each)
- 1 Dry powder inhaler
- 7 Single-use cleaning brushes
- Instructions for Use
Liquidia™
NDC: 72964-014-01
Rx Only106 mcg
per capsuleYutrepia™
(treprostinil)
inhalation powderRead Instructions for Use before each use.
Recommended Dosage: See prescribing information.
Do not remove capsules from blister cards until immediately before use.
Store at 68°F to 77°F (20°C to 25°C); excursions permitted to 59°F to
86°F (15°C to 30°C) [see USP Controlled Room Temperature].
Keep this and all drugs out of the reach of children.Distributed by:
Liquidia Technologies Inc.
Morrisville, NC 27560Liquidia™
-
INGREDIENTS AND APPEARANCE
YUTREPIA
treprostinil capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72964-011 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREPROSTINIL SODIUM (UNII: 7JZ75N2NT6) (TREPROSTINIL - UNII:RUM6K67ESG) TREPROSTINIL 26.5 ug Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LEUCINE (UNII: GMW67QNF9C) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM CHLORIDE (UNII: 451W47IQ8X) Product Characteristics Color YELLOW Score no score Shape CAPSULE Size 16mm Flavor Imprint Code LIQUIDIA;26;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72964-011-01 7 in 1 CARTON 05/23/2025 1 4 in 1 BLISTER PACK 1 1 in 1 CAPSULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213005 05/23/2025 YUTREPIA
treprostinil capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72964-012 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREPROSTINIL SODIUM (UNII: 7JZ75N2NT6) (TREPROSTINIL - UNII:RUM6K67ESG) TREPROSTINIL 53 ug Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LEUCINE (UNII: GMW67QNF9C) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM CHLORIDE (UNII: 451W47IQ8X) Product Characteristics Color GREEN Score no score Shape CAPSULE Size 16mm Flavor Imprint Code LIQUIDIA;53 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72964-012-01 7 in 1 CARTON 05/23/2025 1 4 in 1 BLISTER PACK 1 1 in 1 CAPSULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213005 05/23/2025 YUTREPIA
treprostinil capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72964-013 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREPROSTINIL SODIUM (UNII: 7JZ75N2NT6) (TREPROSTINIL - UNII:RUM6K67ESG) TREPROSTINIL 79.5 ug Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LEUCINE (UNII: GMW67QNF9C) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM CHLORIDE (UNII: 451W47IQ8X) Product Characteristics Color BLUE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code LIQUIDIA;79;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72964-013-01 7 in 1 CARTON 05/23/2025 1 4 in 1 BLISTER PACK 1 1 in 1 CAPSULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213005 05/23/2025 YUTREPIA
treprostinil capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72964-014 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREPROSTINIL SODIUM (UNII: 7JZ75N2NT6) (TREPROSTINIL - UNII:RUM6K67ESG) TREPROSTINIL 106 ug Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LEUCINE (UNII: GMW67QNF9C) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM CHLORIDE (UNII: 451W47IQ8X) Product Characteristics Color PURPLE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code LIQUIDIA;106 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72964-014-01 7 in 1 CARTON 05/23/2025 1 4 in 1 BLISTER PACK 1 1 in 1 CAPSULE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213005 05/23/2025 Labeler - Liquidia Technologies, Inc. (157103164)
Trademark Results [Yutrepia]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 YUTREPIA 98305394 not registered Live/Pending |
Liquidia Technologies, Inc. 2023-12-08 |
 YUTREPIA 98068030 not registered Live/Pending |
Liquidia Technologies, Inc. 2023-06-30 |
 YUTREPIA 88899379 not registered Live/Pending |
Liquidia Technologies, Inc. 2020-05-04 |
 YUTREPIA 88749075 not registered Live/Pending |
Liquidia Technologies, Inc. 2020-01-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.