BB Cream SPF 40 Broad-Spectrum Moisturizer (Cashmere)
Cream by
Drug Labeling and Warnings
Cream by is a Otc medication manufactured, distributed, or labeled by MONAT GLOBAL CORP, NuWorld/Cosmax D&B. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CREAM- zinc oxide cream
MONAT GLOBAL CORP
----------
BB Cream SPF 40 Broad-Spectrum Moisturizer (Cashmere)
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
Directions
- Apply liberally 15 minutes before sun exposure
- Apply to sll skin exposed to the sun
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
-
Sun ProtectionMeasures
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am-2pm
- wear a long-sleeved shirt, pants, hat, and sunglasses
Inactive Ingredients
Water/Eau, Dodecane, Coco-Caprylate, Tricaprylin, Brassica Napus Extract, Coconut Alkanes, Simmondsia Chinensis (Jojoba) Seed Oil, Stearalkonium Bentonite, Polyglceryl-3 Polyricinoleate, Oryza sativa (Rice) Bran Extract, Propanediol, Caprylic/Capric Triglyceride, Glyceryl Caprylate, Coco-caprylate/Caprate, Polyglyceryl-3 Diisostearate, Polyhydroxystearic Acid, Magnesium Sulfate, Dimethicone, Cocos Nucifera (Coconut) Oil, Citrus Limon (Lemon) Peel Oil, Citrus Aurantifolia (Lime) Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Tremella Fuciformis Sporocarp Extract, Camellia Sinensis Leaf Extract, Linoleic Acid, Lecithin, Limnanthes Alba (Meadowfoam) Seed Oil, Crambe Abyssinica Seed Oil, Camellia Oleifera Seed Oil, Solanum Lycopersicum (Tomato) Seed Oil, Daucus Carota Sativa (Carrot) Seed Oil, Phytosteryl Canola Glycerides, Oleic Acid, Palmitic Acid, Stearic Acid, Triolein, Tocopherol, Adansonia Digitata Oil, Mauritia Flexuosa Fruit Oil, Gardenia Taitensis Flower Extract, Moringa Oleifera Seed Oil, Caryocar Brasiliense Fruit Oil, Helianthus Annuus (Sunflower) Seed Oil, Olea Europaea (Olive) Fruit Oil, Simmondsia Chinensis (Jojoba) Seed Extract, Triheptanoin, C9-12 Alkane, Dilinoleic Acid/Butanediol Copolymer, Castor Oil/IPDI Copolymer, Tocopherol Acetate, Ulva Lactuca Extract, Jojoba Esters, Polyglyceryl-6 Polyricinoleate, Silica, Triethyl Citrate, Glycerin, Betain, Glyceryl Undecylenate, Potassium Sorbate, Sodium Benzoate, Bentonite, May Contain: Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499).
| CREAM
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MONAT GLOBAL CORP (027036949) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| COSMAX USA, Corporation | 010990210 | manufacture(78518-002) | |
Trademark Results [Cream]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CREAM 98291021 not registered Live/Pending |
HAPPY SUPPLY LLC 2023-11-29 |
 CREAM 98019827 not registered Live/Pending |
YSMN Inc. 2023-05-30 |
 CREAM 97855230 not registered Live/Pending |
SERVD Distribution 2023-03-24 |
 CREAM 97840280 not registered Live/Pending |
Intellectual Property Holdings LLC 2023-03-15 |
 CREAM 97761211 not registered Live/Pending |
Cream Holdings Limited 2023-01-19 |
 CREAM 97673694 not registered Live/Pending |
Douglas Mahoney 2022-11-11 |
 CREAM 97673239 not registered Live/Pending |
YSMN Inc. 2022-11-11 |
 CREAM 97337744 not registered Live/Pending |
ORTIZ SAMAR, LUIS JOSE 2022-03-30 |
 CREAM 97337744 not registered Live/Pending |
ORTIZ SAMAR, RICARDO 2022-03-30 |
 CREAM 97144845 not registered Live/Pending |
Purry, Richard D. 2021-11-27 |
 CREAM 97002115 not registered Live/Pending |
Nalbandian, Johnny 2021-08-30 |
 CREAM 90770412 not registered Live/Pending |
YSMN Inc. 2021-06-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
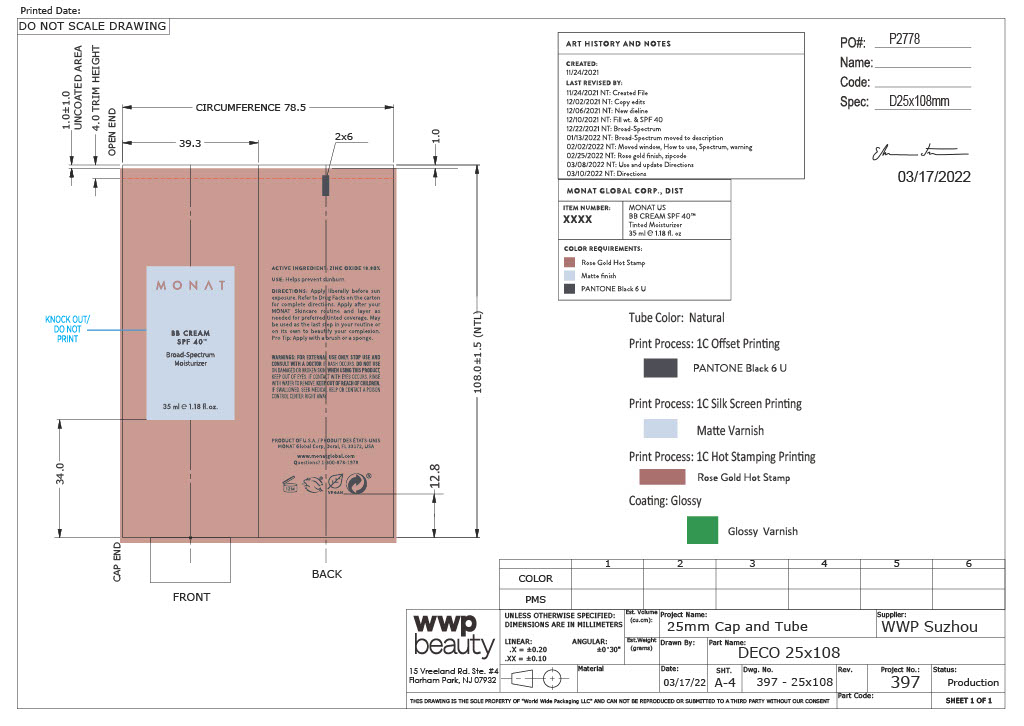 NDC:
NDC:  78518-002-01- Outer
78518-002-01- Outer