NEMLUVIO- nemolizumab-ilto injection, powder, lyophilized, for solution
NEMLUVIO by
Drug Labeling and Warnings
NEMLUVIO by is a Prescription medication manufactured, distributed, or labeled by Galderma Laboratories, L.P., Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Mooswiesen), SHL Medical Assembly & Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NEMLUVIO safely and effectively. See full prescribing information for NEMLUVIO.
NEMLUVIO® (nemolizumab-ilto) for injection, for subcutaneous use
Initial U.S. Approval: 2024RECENT MAJOR CHANGES
INDICATIONS AND USAGE
NEMLUVIO is an interleukin-31 receptor antagonist indicated for:
Prurigo Nodularis
The treatment of adults with prurigo nodularis. (1.1)
Atopic Dermatitis
The treatment of adults and pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis in combination with topical corticosteroids and/or calcineurin inhibitors when the disease is not adequately controlled with topical prescription therapies. (1.2)DOSAGE AND ADMINISTRATION
Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to treatment with NEMLUVIO. (2.1)
Prurigo Nodularis:
- Adult Patients Weighing Less Than 90kg: The recommended subcutaneous dosage is an initial dose of 60 mg (two 30 mg injections), followed by 30 mg given every 4 weeks. (2.2)
- Adult Patients Weighing 90kg or More: The recommended subcutaneous dosage is an initial dose of 60 mg (two 30 mg injections), followed by 60 mg given every 4 weeks. (2.2)
Atopic Dermatitis:
- The recommended subcutaneous dosage is an initial dose of 60 mg (two 30 mg injections), followed by 30 mg given every 4 weeks. (2.3)
- After 16 weeks of treatment, for patients who achieve clear or almost clear skin, a dosage of 30 mg every 8 weeks is recommended. (2.3)
- Use NEMLUVIO with topical corticosteroids and/or topical calcineurin inhibitors. When the disease has sufficiently improved, discontinue use of topical therapies. (2.3)
- Administer NEMLUVIO by subcutaneous injection. (2.5)
- NEMLUVIO must be reconstituted prior to administration. (2.6)
DOSAGE FORMS AND STRENGTHS
For injection: single-dose, prefilled, dual-chamber pen containing 30 mg of nemolizumab-ilto lyophilized powder and diluent, water for injection. (3)
CONTRAINDICATIONS
Known hypersensitivity to nemolizumab-ilto or to any of the excipients in NEMLUVIO. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions are:
- Prurigo Nodularis (incidence ≥1%): headache, dermatitis atopic, eczema, and eczema nummular. (6.1)
- Atopic Dermatitis (incidence ≥1%): headache (including migraine), arthralgia, urticaria, and myalgia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Galderma Laboratories, L.P. at 1-866-735-4137 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.2 Atopic Dermatitis
2 DOSAGE AND ADMINISTRATION
2.1 Vaccination Prior to Treatment
2.2 Recommended Dosage for Prurigo Nodularis
2.3 Recommended Dosage for Atopic Dermatitis
2.4 Missed Dose
2.5 Important Administration Instructions
2.6 Preparation for Use of NEMLUVIO
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Vaccinations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prurigo Nodularis
14.2 Atopic Dermatitis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.2 Atopic Dermatitis
NEMLUVIO is indicated for the treatment of adults and pediatric patients 12 years of age and older with moderate-to-severe atopic dermatitis in combination with topical corticosteroids and/or calcineurin inhibitors when the disease is not adequately controlled with topical prescription therapies.
-
2 DOSAGE AND ADMINISTRATION
2.1 Vaccination Prior to Treatment
Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to treatment with NEMLUVIO [see Warnings and Precautions (5.2)].
2.2 Recommended Dosage for Prurigo Nodularis
Adult Patients Weighing Less Than 90 kg:
The recommended subcutaneous dosage of NEMLUVIO for adult patients weighing less than 90 kg is an initial dose of 60 mg (two 30 mg injections), followed by 30 mg given every 4 weeks.
AdultPatients Weighing 90 kg or More:
The recommended subcutaneous dosage of NEMLUVIO for adult patients weighing 90 kg or more is an initial dose of 60 mg (two 30 mg injections), followed by 60 mg given every 4 weeks.
2.3 Recommended Dosage for Atopic Dermatitis
The recommended subcutaneous dosage of NEMLUVIO in adults and pediatric patients 12 years of age and older is an initial dose of 60 mg (two 30 mg injections), followed by 30 mg given every 4 weeks.
After 16 weeks of treatment, for patients who achieve clear or almost clear skin, a subcutaneous dosage of 30 mg every 8 weeks is recommended.
Concomitant Topical Therapies:
Use NEMLUVIO with topical corticosteroids and/or topical calcineurin inhibitors. When the disease has sufficiently improved, discontinue use of topical therapies.2.4 Missed Dose
If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regular scheduled time.
2.5 Important Administration Instructions
- NEMLUVIO is administered by subcutaneous injection.
- NEMLUVIO is intended for use under the guidance of a healthcare provider. Prior to the first injection, provide patients and/or caregivers with proper training on the preparation and administration of NEMLUVIO. Patients may self-inject NEMLUVIO after receiving training on subcutaneous injection techniques. In pediatric patients 12 years of age and older, administer NEMLUVIO by or under the supervision of a trained adult or caregiver.
- For the initial dose, administer each of the two NEMLUVIO injections at different injection sites.
- Administer NEMLUVIO subcutaneously into the front upper thighs or abdomen avoiding the 2-inch (5 cm) area around the navel. NEMLUVO may be subcutaneously injected into the upper arm, but this should only be performed by a caregiver or healthcare professional.
- Alternate the injection site with each injection. Do not inject NEMLUVIO into skin that is tender, inflamed, swollen, damaged or has bruises or scars or open wounds.
- Refer to the Instructions for Use for complete administration instructions with illustrations [see Instructions for Use].
2.6 Preparation for Use of NEMLUVIO
- Before injection, remove NEMLUVIO carton from the refrigerator and allow to reach room temperature (30-45 minutes).
- Inspect NEMLUVIO visually prior to reconstitution. NEMLUVIO is supplied in a single-dose, prefilled, dual-chamber pen with white powder in one chamber and a clear diluent in the other chamber. Do not use if powder is not white, or if diluent is cloudy or contains visible particles.
- NEMLUVIO must be reconstituted prior to administration. Refer to the Instructions for Use for complete preparation instructions with illustrations [see Instructions for Use].
- Following reconstitution, each prefilled pen delivers 30 mg/0.49 mL as a clear and colorless to slightly yellow solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the reconstituted solution has discoloration or contains particles.
- Use NEMLUVIO pens within 4 hours after reconstitution. Discard unused reconstituted NEMLUVIO pens after 4 hours.
- Discard any unused portions after administration.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
NEMLUVIO is contraindicated in patients who have known hypersensitivity to nemolizumab-ilto or to any of the excipients in NEMLUVIO [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity reactions, such as facial angioedema, have been reported with use of NEMLUVIO. NEMLUVIO is contraindicated in patients with a known hypersensitivity to nemolizumab-ilto or to any of the excipients in NEMLUVIO. If a clinically significant hypersensitivity reaction occurs, immediately institute appropriate therapy and discontinue NEMLUVIO [see Contraindications (4), Adverse Reactions (6.1)].
5.2 Vaccinations
Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to treatment with NEMLUVIO. Avoid use of live vaccines in patients during treatment with NEMLUVIO. It is unknown if administration of live vaccines during NEMLUVIO treatment will impact the safety or effectiveness of these vaccines. No data are available on the response to non-live vaccines.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in greater details elsewhere in the labeling:
- Hypersensitivity [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Prurigo Nodularis
A total of 508 adult subjects with prurigo nodularis were treated with NEMLUVIO in two placebo-controlled trials and an open label long-term extension trial. Of these, 375 subjects were exposed for at least 1 year in the drug development program for prurigo nodularis.The safety of NEMLUVIO in adult subjects with prurigo nodularis was evaluated in two randomized, doubleblind, placebo-controlled, multicenter trials (OLYMPIA 1 and OLYMPIA 2). Subjects were treated for up to 24 weeks in OLYMPIA 1 and up to 16 weeks in OLYMPIA 2. In these 2 trials, 370 subjects were treated with subcutaneous injections of NEMLUVIO, and 186 subjects received placebo [see Clinical Studies (14.1)].
Subjects weighing less than 90 kg in the NEMLUVIO group received an initial NEMLUVIO dose of 60 mg or placebo at Week 0, followed by a NEMLUVIO dose of 30 mg or placebo every 4 weeks. Subjects weighing 90 kg or more in the NEMLUVIO group received an initial NEMLUVIO dose of 60 mg or placebo at Week 0 followed by a NEMLUVIO dose of 60 mg or placebo every 4 weeks.
During the treatment period in Trials OLYMPIA 1 and OLYMPIA 2, the proportion of subjects who discontinued treatment because of adverse reactions was 4% in the NEMLUVIO group versus 3% in the placebo group. Table 1 summarizes the adverse reactions that occurred at a rate of at least 1% in the NEMLUVIO group, and for which the rate exceeds the rate in the placebo group through Week 16.
Table 1: Adverse Reactions Occurring in ≥1% of Adult Subjects with Prurigo Nodularis in the NEMLUVIO Group and Greater than Placebo in the OLYMPIA 1 and OLYMPIA 2 Trials through Week 16. *includes: headache and tension headache Adverse Reaction NEMLUVIO
N= 370
n (%)Placebo
(N= 186)
n (%)Headache* 23 (6) 6 (3) Dermatitis atopic 16 (4)
1 (0.5) Eczema
14 (4)
3 (2) Eczema nummular
11 (3) 0 Specific Adverse Reactions
Hypersensitivity reactions
Type 1 hypersensitivity reactions (Ig-E mediated reactions), including one report of discrete facial (peri-ocular) angioedema, were reported in subjects treated with NEMLUVIO [see Contraindications (4)].Atopic Dermatitis
Adults and Pediatric Subjects 12 Years of Age and Older
A total of 1148 subjects, including 180 subjects 12 to 17 years of age, with moderate-to-severe atopic dermatitis were treated with NEMLUVIO for at least 1 year during the drug development program.The safety of NEMLUVIO was evaluated in a pool of two randomized, double-blind, placebo-controlled, multicenter phase 3 trials (ARCADIA 1, ARCADIA 2). In these two trials, 1135 adult and pediatric subjects 12 years of age and older with moderate-to-severe AD were treated with subcutaneous injections of NEMLUVIO (initial dose of 60 mg, followed by 30 mg every 4 weeks), with concomitant topical corticosteroids (TCS) and/or topical calcineurin inhibitors (TCI) for up to 16 weeks (Initial Treatment Period) [see Clinical Studies (14.2)].
After the Initial Treatment Period, subjects who responded to NEMLUVIO were re-randomized to receive NEMLUVIO 30 mg every 4 weeks, NEMLUVIO 30 mg every 8 weeks, or placebo every 4 weeks for the Maintenance Treatment Period (Week 16 through Week 48) [see Clinical Studies (14.2)]. The safety population during the Maintenance Treatment Period had a mean age of 31 to 33 years (median age of 26 to 29 years) for the NEMLUVIO cohorts.
Week 0 to Week
16 In ARCADIA 1 and ARCADIA 2 trials through Week 16, the proportion of subjects who discontinued treatment because of adverse events was 2.3% in the NEMLUVIO every 4 weeks group and 2.2% in the placebo groups. Table 2 summarizes the adverse reactions that occurred at a rate of at least 1% in the NEMLUVIO group, and for which the rate exceeds the rate in the placebo group during the first 16 weeks of treatment.Table 2: Adverse Reactions Occurring in ≥1% of Adult and Pediatric Subjects 12 Years of Age and Older with Atopic Dermatitis in the NEMLUVIO Group and Greater than in the Placebo Group in ARCADIA 1 and ARCADIA 2 Trials through Week 16 Adverse reactions NEMLUVIO
N = 1135
n (%)Placebo
N=584
n (%)Headache (incl. migraine)
52 (5) 22 (4) Arthralgia
12 (1) 1 (0.2) Urticaria 12 (1) 2 (0.3) Myalgia 11 (1) 1 (0.2) Assessment of the safety profile of NEMLUVIO in 505 subjects up through Week 48 in the ARCADIA 1 and ARCADIA 2 trials was generally consistent with the safety profile observed at Week 16.
Specific Adverse Reactions
Hypersensitivity reactions
Type 1 hypersensitivity reactions (Ig-E mediated reactions) were reported in subjects treated with NEMLUVIO. This included occurrence of mild urticaria that did not lead to discontinuation of treatment.Injection site reactions
The incidence of injection site reactions during the initial treatment period was reported for 1.2% of subjects treated with NEMLUVIO and 0.9% of subjects receiving placebo; during the maintenance period, the incidence was 0.6% with NEMLUVIO every 4 weeks, 0% with NEMLUVIO every 8 weeks, and 0% with placebo. None of these reactions led to discontinuation of treatment.Herpes Zoster
During the initial treatment period, herpes zoster infections were reported in 5 subjects (0.4%) treated with NEMLUVIO (including 1 case of ophthalmic herpes zoster) and no subjects receiving placebo. Cases of herpes zoster were mild to moderate in severity and did not lead to discontinuation of treatment.Pediatric Subjects 12 Years of Age and Older
The safety of NEMLUVIO was assessed in 176 subjects 12 to 17 years of age with moderate-to-severe atopic dermatitis enrolled in the ARCADIA 1 or ARCADIA 2 trials who received at least one dose of NEMLUVIO from Week 0 to Week 16 in the primary safety population. The safety profile of NEMLUVIO in these subjects through Week 16 was consistent with the safety profile observed in adults with atopic dermatitis.The safety profile of NEMLUVIO in 98 subjects 12 to 17 years of age followed through Week 48 was consistent with the safety profile observed at Week 16.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data on NEMLUVIO use in pregnant women exposed during clinical trials are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In an enhanced pre- and postnatal development study in cynomolgus monkeys, when nemolizumab-ilto was administered subcutaneously during organogenesis to parturition, an increase in early postnatal death was observed at a dose 36 times the maximum recommended human dose (MRHD) for PN and 50 times for AD (see Data). The clinical significance of this nonclinical finding is unknown.
The background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.Clinical Considerations
Fetal/Neonatal Adverse Reactions
Transport of endogenous IgG antibodies across the placenta increases as pregnancy progresses, and peaks during the third trimester. Therefore, NEMLUVIO may be present in infants exposed in utero. The potential impact of infants being exposed in utero to NEMLUVIO should be considered.Data
Animal Data
In an enhanced pre- and postnatal development study, subcutaneous doses up to 25 mg/kg of nemolizumab-ilto were administered to pregnant cynomolgus monkeys once every two weeks during organogenesis to parturition. No maternal or embryofetal toxicities were observed at doses up to 25 mg/kg (36 times the MRHD for PN and 50 times for AD, based on AUC comparison). Early postnatal death occurred in the offspring of one control monkey and 3 monkeys at 25 mg/kg (36 times the MRHD for PN and 50 times for AD, based on AUC comparison). The clinical significance of this nonclinical finding is unknown. Nemolizumab-ilto was administered subcutaneously to the offspring at doses up to 25 mg/kg (122 times the MRHD for PN and 168 times for AD, based on AUC comparison), once every 2 weeks for 6 months, starting from postnatal day 35. No adverse effects were noted in the remaining offspring.8.2 Lactation
Risk Summary
There are no data on the presence of nemolizumab-ilto in human milk, the effects on the breastfed infant, or the effects on milk production. Nemolizumab-ilto was detected in breast milk of monkeys (see Data). Endogenous maternal IgG and monoclonal antibodies are transferred in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to nemolizumab-ilto are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NEMLUVIO and any potential adverse effects on the breastfed child from NEMLUVIO or from the underlying maternal condition.Data
Nemolizumab-ilto was detected in breast milk of monkeys in the enhanced pre- and postnatal development study following subcutaneous doses up to 25 mg/kg once every two weeks during organogenesis to parturition. The mean nemolizumab-ilto concentrations in milk were approximately 0.3 – 0.5% of the maternal plasma levels from lactation day 7 to 63. The concentration of nemolizumab-ilto in animal milk does not necessarily predict the concentration of drug in human milk.8.4 Pediatric Use
Prurigo Nodularis
The safety and effectiveness of NEMLUVIO have not been established in pediatric patients.Atopic Dermatitis
The safety and effectiveness of NEMLUVIO for the treatment of moderate-to-severe atopic dermatitis in combination with topical corticosteroids and/or calcineurin inhibitors have been established in pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies. Use of NEMLUVIO for this indication is supported by evidence from two randomized, double-blind, placebo-controlled trials [See Adverse Reactions (6.1) and Clinical Studies (14.2)].
The safety and effectiveness of NEMLUVIO have not been established in pediatric patients younger than 12 years of age.8.5 Geriatric Use
Prurigo Nodularis
Of the 370 subjects with prurigo nodularis exposed to NEMLUVIO in OLYMPIA 1 and OLYMPIA 2, 99 (26.8%) subjects were 65 years of age or older. The long-term safety of NEMLUVIO was assessed in 508 subjects, among which 133 (26.2%) were 65 years of age or older. Clinical trials of NEMLUVIO did not include sufficient numbers of subjects 65 years of age or older to determine whether they respond differently than younger adult subjects. [see Clinical Pharmacology (12.3)].Atopic Dermatitis
Of the 1192 subjects with atopic dermatitis exposed to NEMLUVIO in the primary safety population, 72 (6.0%) subjects were 65 years of age or older. The long-term safety of NEMLUVIO was assessed in 78 (4.5%) subjects 65 years of age or older. Clinical studies of NEMLUVIO did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger adult subjects [see Clinical Pharmacology (12.3)]. - 10 OVERDOSAGE
-
11 DESCRIPTION
Nemolizumab-ilto, an interleukin-31 receptor alpha (IL-31RA) antagonist, is a humanized monoclonal modified immunoglobulin G (IgG) antibody with a molecular weight of approximately 144 kDa. Nemolizumab-ilto is produced by recombinant DNA technology in Chinese Hamster Ovary cells.
NEMLUVIO (nemolizumab-ilto) for injection is a sterile, preservative-free, white lyophilized powder in a dualchamber, single-dose, prefilled pen for subcutaneous use. One chamber contains 30 mg of nemolizumab-ilto with inactive ingredients arginine hydrochloride (9.5 mg), poloxamer 188 (0.15 mg), sucrose (25.8 mg), trometamol (0.10 mg), and tris hydrochloride for pH adjustment. The diluent, water for injection, is in the other chamber. Following reconstitution, each prefilled pen delivers 30 mg/0.49 mL of nemolizumab-ilto with a pH of 6.7 to 7.3.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nemolizumab-ilto is a humanized IgG2 monoclonal antibody that inhibits IL-31 signaling by binding selectively to IL-31 RA. IL-31 is a naturally occurring cytokine that is involved in pruritus, inflammation, epidermal dysregulation, and fibrosis. Nemolizumab-ilto inhibited IL-31-induced responses including the release of proinflammatory cytokines and chemokines.
12.2 Pharmacodynamics
Pharmacodynamics of nemolizumab-ilto in the treatment of prurigo nodularis and atopic dermatitis are unknown.
12.3 Pharmacokinetics
After a single-dose, nemolizumab-ilto exposure increased dose proportionally over a dose range of 0.03 and 3 mg/kg following subcutaneous administration. After multiple doses, nemolizumab-ilto systemic exposure increased in an approximately dose-proportional manner across the subcutaneous dose range up to 30 mg. There was a decrease in bioavailability by 9% with the 60 mg subcutaneous dose and by 15% with the 90 mg subcutaneous dose.
- Prurigo Nodularis: Following multiple doses of NEMLUVIO in subjects with prurigo nodularis, the estimated mean (SD) steady-state trough concentrations of nemolizumab-ilto were 3.04 (1.23) μg/mL in subjects with bodyweight less than 90 kg; and 3.66 (1.63) μg/mL in subjects with bodyweight of 90 kg or more. Steady state nemolizumab-ilto concentrations were achieved by week 4 in subjects weighting less than 90 kg and by week 12 in subjects weighing 90 kg or more.
- Atopic Dermatitis: Following multiple doses of NEMLUVIO in subjects with atopic dermatitis, the estimated mean (SD) steady-state trough concentrations of nemolizumab-ilto were 2.63 (1.27) μg/mL for 30 mg administered every 4 weeks and 0.74 (0.44) μg/mL for 30 mg administered every 8 weeks. Steady-state nemolizumab-ilto concentrations were achieved 4 weeks after the initial 60 mg loading dose.
Absorption
Following an initial subcutaneous dose of 60 mg, nemolizumab-ilto reached peak mean (SD) concentrations (Cmax) of 7.5 (2.31) μg/mL by approximately 6 days post dose.Distribution
The volume of distribution of nemolizumab-ilto was estimated to be 7.67 L.Elimination
Nemolizumab-ilto is expected to be degraded in the same manner as endogenous IgG. The terminal elimination half-life (SD) of nemolizumab-ilto was estimated to be 18.9 (4.96) days and systemic clearance was estimated to be 0.263 L/day.Metabolism
The metabolic pathway of nemolizumab-ilto has not been characterized. Nemolizumab-ilto is expected to be degraded into small peptides by catabolic pathways.Specific Populations
Geriatric Populations
No clinically significant difference in the pharmacokinetics of nemolizumab-ilto was estimated based on age (subjects 18 to 65 years of age and older than 65 years of age). Dose adjustment in this population is not needed.
Pediatric Population
- Atopic Dermatitis: No clinically significant difference in the pharmacokinetics of nemolizumab-ilto was estimated in pediatric subjects 12 to 17 years of age compared to adults. Dose adjustment in this population is not needed.
Body Weight
The exposure of nemolizumab-ilto decreases with increasing body weight. After a 30-mg dose every 4 weeks, the steady state mean exposure parameters (AUCss, Cmaxss and Ctrough) of subjects with bodyweight of above 87 kg is expected to be 1.7-fold lower than that of subjects weighing below 62 kg.-
Prurigo Nodularis: The variability in systemic exposure due to body weight had a clinically meaningful impact on skin lesion efficacy (IGA response) but not on pruritus improvement.
Atopic Dermatitis: The difference in systemic exposure due to body weight had no clinically meaningful impact on efficacy in subjects with atopic dermatitis. Dose adjustment based on body weight is not needed.
Drug Interaction Studies
The effects of nemolizumab on the pharmacokinetics of midazolam (CYP3A4/5 substrate), warfarin (CYP2C9 substrate), omeprazole (CYP2C19 substrate), metoprolol (CYP2D6 substrate), and caffeine (CYP1A2 substrate) were evaluated in a study in 14 subjects with moderate to severe AD receiving an initial SC dose of 60 mg followed by 30 mg SC every four weeks for 12 weeks. No clinically significant changes in the exposure of CYP450 substrates before and after multiple nemolizumab injections were observed, with Cmax and AUC ratios ranging from 88.2 to 107.8%. The concomitant use of nemolizumab-ilto is unlikely to influence the PK profiles of CYP substrates.12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of nemolizumab-ilto or other nemolizumab products.
Prurigo Nodularis:
- In the Phase 3 trials (OLYMPIA 1, OLYMPIA 2) up to 24 weeks, the incidence of treatment-emergent ADAs was 7%; neutralizing antibodies were seen in 3% of subjects.
Atopic Dermatitis:
- In the phase 3 trials (ARCADIA 1, ARCADIA 2) up to 16 weeks, the incidence of treatment-emergent ADAs was 6.1% (67/1092). Among subjects who continued treatment from week 16 through week 48 in the two studies, the ADA incidence was 13.9% (23/166) for those that received 30 mg every 4 weeks, and 10.6% (17/161) for those that received 30 mg every 8 weeks. Neutralizing antibody incidence was 4.5% (3/67) among the ADA+ subjects throughout 48 weeks in ARCADIA 1 and ARCADIA 2, respectively.
Anti-Drug Antibody Effects on Pharmacokinetics and Pharmacodynamics
-
Prurigo Nodularis: In the phase 3 trials in subjects with prurigo nodularis, there was no identified clinically significant effect of anti-drug antibodies on the pharmacokinetics, safety or efficacy of nemolizumab-ilto over the treatment duration of 24 weeks.
- Atopic Dermatitis: In the phase 3 trials in subjects with atopic dermatitis, antibodies to nemolizumab-ilto were associated with reduced serum nemolizumab-ilto concentrations beyond Week 16. NEMLUVIO treated subjects who developed ADAs had nemolizumab-ilto concentrations that were 20% to 70% lower compared to subjects who did not develop ADAs. There was no clinically significant effect of anti-drug antibodies on safety or efficacy of nemolizumab-ilto over the treatment duration of 48 weeks.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of NEMLUVIO.
No effects on fertility parameters as reproductive organ morphology, menstrual cycle length, or sperm/testicular analysis were observed in male or female sexually mature cynomolgus monkeys that were administered nemolizumab-ilto at subcutaneous doses up to 25 mg/kg once every two weeks for 6 months (53 times the MRHD for PN and 72 times for AD, based on AUC comparison). The monkeys were not mated to evaluate fertility. -
14 CLINICAL STUDIES
14.1 Prurigo Nodularis
Two randomized, double-blind, placebo-controlled trials (OLYMPIA 1 [NCT04501666] and OLYMPIA 2 [NCT04501679]) enrolled a total of 560 adult subjects with prurigo nodularis (PN). Disease severity was defined using an Investigator’s Global Assessment (IGA) in the overall assessment of prurigo nodularis nodules on a severity scale of 0 to 4. Subjects enrolled in these two trials had an IGA score ≥ 3, severe pruritus as defined by a weekly average of the peak pruritus numeric rating scale (PP-NRS) score of ≥7 on a scale of 0 to 10, and greater than or equal to 20 nodular lesions. OLYMPIA 1 and OLYMPIA 2 assessed the effect of NEMLUVIO on the signs and symptoms of PN, targeting improvement in skin lesions and pruritus over 16 weeks. In OLYMPIA 1, subjects were extended up to 24 weeks of treatment.
Subjects weighing less than 90 kg in the NEMLUVIO group received subcutaneous injections of NEMLUVIO 60 mg at Week 0, followed by NEMLUVIO 30 mg injections every 4 weeks. Subjects weighing 90 kg or more in the NEMLUVIO group received subcutaneous injections of NEMLUVIO 60 mg at Week 0 and every 4 weeks.
In these trials, at baseline, 60% of subjects were female, 81% were White, 9% were Asian, 7% were Black or African American; for ethnicity, 4% of subjects identified as Hispanic or Latino. Twenty-five (25)% of subjects were older than 65 years of age. Thirty-two (32)% of subjects had a history of atopy. The baseline weekly average PP-NRS score was a mean of 8.5. Fifty-eight (58)% of subjects had a baseline IGA score of 3 (moderate PN), and 42% of subjects had a baseline IGA of 4 (severe PN).
The PP-NRS score is a weekly average of daily PP-NRS scores on an 11-point scale from 0-10 that assesses the maximal intensity of pruritus in the last 24 hours with 0 being no itch and 10 being worst itch imaginable. The IGA is a 5-category scale, including “0 = clear”, “1 = almost clear”, “2 = mild”, “3 = moderate” or “4 = severe” indicating the investigator’s overall assessment of the pruriginous nodules.
Efficacy was assessed with the proportion of subjects with an improvement of ≥4 from baseline in PP-NRS, the proportion of subjects with an IGA of 0 (Clear) or 1 (Almost Clear) and a ≥2-point improvement from baseline, the proportion of subjects who achieved a response in both PP-NRS and IGA per the criteria described above, and the proportion of subjects with PP-NRS <2.
The efficacy results for OLYMPIA 1 and OLYMPIA 2 are presented in Table 3 and Figures 1, 2, and 3.
Table 3: Efficacy Results at Week 16 in Adult Subjects with PN in OLYMPIA1 and OLYMPIA 2 a Not adjusted for multiplicity.
b Subjects who received rescue therapy or had missing data (fewer than 4 PP-NRS daily diary entries in a 7-day period) were considered non-responders.
OLYMPIA 1 OLYMPIA2 NEMLUVIO
(N=190)
Placebo
(N=96)Difference from Placebo
(95% CI)NEMLUVIO
(N=183)Placebo
(N=91)Difference from Placebo
(95% CI)Proportion of subjects with both an improvement (reduction) of ≥4 from baseline in PP-NRS and IGA 0 or 1a,b 22%a 2%a 15%
(8%, 21%)a25%a 4%a 22%
(14%, 30%)aProportion of subjects with IGA 0 or 1b 26% 7% 15%
(7%, 23%)38% 11% 29%
(19%, 38%)Proportion of subjects with an improvement (reduction) of ≥4 from baseline in PP-NRSb 56% 16% 38%
(27%, 48%)49% 16% 34%
(23%, 45%)Proportion of subjects with PP-NRS <2b 32% 4% 28%
(20%, 36%)31% 7% 26%
(18%, 34%)Figure 1: Proportion of Adult Subjects with PN with PP-NRS Improvement ≥4 from Baseline Over Time in OLYMPIA 1 and OLYMPIA 2a

a Subjects who received rescue therapy or had missing data (fewer than 4 PP-NRS daily diary entries in a 7-day period) were considered non-responders.
Figure 2: Proportion of Adult Subjects with PN with PP-NRS <2 Over Time in OLYMPIA 1 and OLYMPIA 2a

a Subjects who received rescue therapy or had missing data (fewer than 4 PP-NRS daily diary entries in a 7-day period) were considered non-responders.
Figure 3: Proportion of Adult Subjects with PN with IGA Responsea Over Time in OLYMPIA 1 and OLYMPA 2b
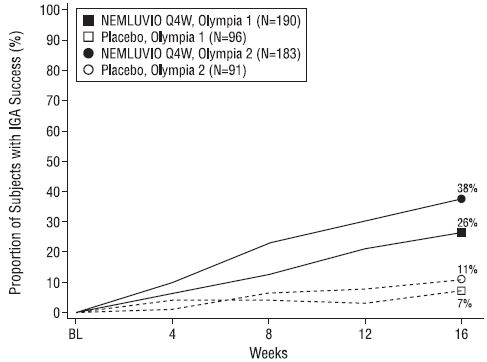
a Response was defined as an IGA of 0 (Clear) or 1 (Almost Clear) and a ≥2-point improvement from baseline.
b Subjects who received rescue therapy or had missing data were considered non-responders.Examination of weight, age, gender, race, history of atopy, and prior treatment did not identify meaningful differences in response to NEMLUVIO among these subgroups at Week 16.
14.2 Atopic Dermatitis
Two randomized, double-blind, placebo-controlled trials (ARCADIA 1 [NCT03985943] and ARCADIA 2 [NCT03989349]) enrolled a total of 1728 subjects 12 years of age and older with moderate-to-severe atopic dermatitis not adequately controlled by topical treatments. Disease severity was defined by an Investigator’s Global Assessment (IGA) score of 3 (moderate) and 4 (severe) in the overall assessment of atopic dermatitis, an Eczema Area and Severity Index (EASI) score of ≥16, a minimum body surface area (BSA) involvement of ≥10%, and a Peak Pruritus Numeric Rating Scale (PP-NRS) score of ≥ 4.
During the initial 16-week treatment period, subjects in the NEMLUVIO group received an initial subcutaneous injection of NEMLUVIO 60 mg (two 30 mg injections), followed by 30 mg injections every 4 weeks. Concomitant low and/or medium potency TCS and/or TCI were administered for at least 14 days prior to baseline and continued during the trial. Based on disease activity, these concomitant therapies could be tapered and/or discontinued at investigator discretion.
After 16 weeks, subjects achieving either EASI-75 or IGA success (i.e. NEMLUVIO responders) continued into the trial maintenance period for another 32 weeks to evaluate the maintenance of response achieved at Week 16. NEMLUVIO responders were re-randomized to receive either NEMLUVIO 30 mg every 4 weeks, NEMLUVIO 30 mg every 8 weeks, or placebo every 4 weeks (all groups continued background TCS/TCI). Subjects randomized to placebo in the initial treatment period who achieved the same clinical response at Week 16 continued to receive placebo every 4 weeks.
In these trials, at baseline, 51% of subjects were male, 80% were White, 13% were Asian, and 6% were Black or African American; for ethnicity, 9% of subjects identified as Hispanic or Latino. Fifteen (15)% of subjects were 12-17 years of age. Seventy (70)% of subjects had a baseline IGA score of 3 (moderate AD), and 30% of subjects had a baseline IGA score of 4 (severe AD). The baseline mean EASI score was 27.5 and the baseline mean weekly average PP-NRS was 7.1. Overall, 63% of subjects received other previous systemic treatments for AD.
The IGA is a 5-category scale, including “0 = clear”, “1 = almost clear”, “2 = mild”, “3 = moderate” or “4 = severe” indicating the investigator’s overall assessment of the AD. EASI scores range from 0 to 72 points and reflect the severity and extent of AD. EASI-75 indicates at least a 75% improvement in EASI score from baseline. The PP-NRS score is a weekly average of daily PP NRS scores on an 11-point scale from 0-10 that assesses the maximal intensity of pruritus in the last 24 hours with 0 being no itch and 10 being worst itch imaginable.
Both ARCADIA 1 and ARCADIA 2 assessed the co-primary endpoints of:
- Proportion of subjects with an IGA success (defined as an IGA of 0 [clear] or 1 [almost clear] and a ≥2- point reduction from baseline) at Week 16
- Proportion of subjects with EASI-75 (≥75% improvement in EASI from baseline) at Week 16
PP-NRS improvement ≥ 4 from baseline at Week 16 was a key secondary outcome in both trials.
Clinical Response at Week 16 (ARCADIA 1 and ARCADIA 2)
The efficacy results for ARCADIA 1 and ARCADIA 2 evaluating the initial treatment period with NEMLUVIO administered every 4 weeks over 16 weeks are presented in Table 4.Table 4. Efficacy Results of NEMLUVIO (Every 4 Weeks) with Concomitant TCS/TCI at Week 16 in Adult and Pediatric Subjects 12 Years of Age and Older with Moderate to Severe AD in ARCADIA 1 and ARCADIA 2 a Subjects who received rescue treatment or had missing data (fewer than 4 PP-NRS daily diary entries in a 7-day period) were considered non-responders. ARCADIA 1 ARCADIA 2 NEMLU
VIO
Every 4 Weeks
+ TCS/TCIPlacebo + TCS/TCI Difference
from
Placebo
(95% CI)NEMLUVIO
Every
4 Weeks
+ TCS/TCIPlacebo +
TCS/TCIDifference
from
Placebo
(95% CI)Number of subjects
randomized620 321 522 265 Proportion of subjects with IGA 0 or 1a 36% 25% 12%
(6%, 17%)
38% 26% 12%
(6%, 19%)Proportion of subjects with EASI-75a 44% 29% 15%
(9%, 21%)42% 30% 12%
(6%, 19%)Proportion of subjects with an improvement (reduction) of ≥4 from baseline in PP-NRSa
33%
15%
18%
(13%, 23%)36%
15%
21%
(15%, 27%)
Examination of weight, age, gender, race, and prior treatment did not identify meaningful difference in response to NEMLUVIO among these subgroups at Week 16.
Maintenance and Durability of Response (Week 16 to Week 48)
The maintenance and durability of response in NEMLUVIO responders (IGA 0/1 or EASI-75 at Week 16) was evaluated between Week 16 and Week 48 in ARCADIA 1 and ARCADIA 2 trials. For the maintenance treatment period, NEMLUVIO responders were re-randomized to NEMLUVIO 30 mg every 4 weeks, NEMLUVIO 30 mg every 8 weeks or placebo every 4 weeks (NEMLUVIO withdrawal) with concomitant TCS/TCI. The results are presented in Table 5.Table 5. Efficacy Results of NEMLUVIO with Concomitant TCS/TCI at Week 48 in Adult and Pediatric Subjects 12 Years of Age and Older with Moderate to Severe AD in ARCADIA 1 and ARCADIA 2 a Responder was defined as a subject with an IGA of 0 (clear) or 1 (almost clear) and a ≥ 2-point reduction from baseline.
b Subjects who received rescue treatment or with missing data were considered non-responders.NEMLUVIO
Every 4 Weeks
+ TCS/TCINEMLUVIO
Every 8 Weeks
+ TCS/TCIPlacebo +
TCS/TCINumber of subjects who were IGA
Respondersa at Week 16142 142 131 Proportion of subjects with IGA 0 or
1b at Week 4863% 64% 55% Number of subjects who were
EASI-75 Responders at Week 16163 163 157 Proportion of subjects with EASI-75b
at Week 4875% 77% 65% -
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
NEMLUVIO (nemolizumab-ilto) for injection is a sterile, preservative-free, white lyophilized powder available in a single-dose, dual-chamber, prefilled pen containing 30 mg of nemolizumab-ilto in one chamber and the diluent, water for injection, in the other chamber. Following reconstitution, each prefilled pen delivers 30 mg/0.49 mL of nemolizumab-ilto.Each carton contains 1 single-dose prefilled pen:
Presentation Pack size NDC# Prefilled Pen Pack of 1 pen 0299-6220-15 Storage and Handling
Store the NEMLUVIO dual-chamber, prefilled pen in a refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light until the expiration date. Do not freeze. Do NOT expose to heat or direct sunlight.Alternatively, the NEMLUVIO carton containing the unused dual-chamber, prefilled pen may be stored at room temperature [up to 77°F (25°C)] for up to 90 days. Write the date the NEMLUVIO dual-chamber, prefilled pen is first removed from the refrigerator in the space provided on the inner partition for the pen. Do not use the NEMLUVIO dual-chamber, prefilled pen beyond the expiration date or 90 days after the date it was first removed from the refrigerator (whichever is earlier).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity
Advise patients to seek immediate medical attention and discontinue NEMLUVIO if they experience any symptoms of hypersensitivity reactions [see Warnings and Precautions (5.1)].
Vaccinations
Instruct patients to inform their healthcare provider that they are taking NEMLUVIO prior to a potential vaccination [see Warnings and Precautions (5.2)].
Administration Instructions:- Instruct patients and/or caregivers to receive proper training in subcutaneous injection technique prior to self-injection [see Dosage and Administration (2.5)]. Inform patients and/or caregivers that Galderma Customer Support may be called toll-free for assistance at 1-866-735-4137.
- Inform patients that NEMLUVIO must be reconstituted prior to administration. Advise patients and/or caregivers to refer to the Instructions for Use that accompanies the NEMLUVIO pen for complete mixing and administration instructions with illustrations [see Dosage and Administration (2.5, 2.6), Instructions for Use].
- Inform patients and/or caregivers of proper pen disposal and caution against any reuse of needles. Instruct patients and/or caregivers to discard used pens in an appropriate sharps disposal container following safe needle disposal practices[see Instructions for Use].
- Advise patients and/or caregivers of the importance of complying with dosing schedule. If a dose is missed, instruct patients and/or caregivers to administer the injection as soon as possible, and thereafter, resume dosing at the regular scheduled time [see Dosage and Administration (2.4)].
Manufactured by:
Galderma Laboratories, L.P., Dallas, TX 75201
U.S. License No. 2289
NEMLUVIO® is a registered trademark of Galderma.
© 2025 Galderma Laboratories, L.P. All rights reserved.
-
INFORMATION FOR PATIENTS
PATIENT INFORMATION
NEMLUVIO® [Nem LOO vee oh]
(nemolizumab-ilto)
for injection, for subcutaneous useWhat is NEMLUVIO?
NEMLUVIO is a prescription medicine used:
- to treat adults with prurigo nodularis.
- to treat adults and children 12 years of age and older with moderate-to-severe eczema (atopic dermatitis) that is not well controlled with prescription therapies used on the skin (topical). NEMLUVIO can be used with certain prescription topical medicines.
It is not known if NEMLUVIO is safe and effective in children with prurigo nodularis under 18 years of age.
It is not known if NEMLUVIO is safe and effective in children with atopic dermatitis under 12 years of age.Do not take NEMLUVIO if you are allergic to nemolizumab-ilto or to any ingredients in NEMLUVIO. See the end of this Patient Information leaflet for a complete list of ingredients in NEMLUVIO.
Before taking NEMLUVIO, tell your healthcare provider about all of your medical conditions, including if you:
- are scheduled to receive any vaccination. You should avoid receiving a live vaccine right before or during treatment with NEMLUVIO.
- are pregnant or plan to become pregnant. It is not known whether NEMLUVIO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known whether NEMLUVIO passes into your breast milk and if it can harm your baby.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take NEMLUVIO?
- See the detailed Instructions for Use that comes with NEMLUVIO for information on how to prepare and inject NEMLUVIO and how to properly store and throw away (dispose of) used NEMLUVIO prefilled pens.
- Use NEMLUVIO exactly as prescribed by your healthcare provider.
- Use NEMLUVIO with prescription topical therapies. When your eczema has sufficiently improved, your healthcare provider may discontinue topical therapies.
- Your healthcare provider will tell you how much NEMLUVIO to inject and how often to inject it.
- NEMLUVIO comes as a single-dose prefilled pen with a needle guard.
- NEMLUVIO is given as an injection under the skin (subcutaneous injection).
- If your healthcare provider decides that you or a caregiver can give the injections of NEMLUVIO, you or your caregiver should receive training on the right way to prepare and inject NEMLUVIO. Do not try to inject NEMLUVIO until you have been shown the right way by your healthcare provider.
- If you miss a dose, inject the missed dose as soon as possible, then continue with your next dose at your regular scheduled time.
- If you inject too much NEMLUVIO, call your healthcare provider or the Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
- Your healthcare provider may prescribe other medicines to use with NEMLUVIO. Use the other prescribed medicines exactly as your healthcare provider tells you to.
What should I avoid while taking NEMLUVIO?
You should avoid receiving live vaccines while taking NEMLUVIO.What are the possible side effects of NEMLUVIO?
NEMLUVIO may cause serious side effects, including:
- allergic reactions (hypersensitivity). NEMLUVIO can cause allergic reactions that can sometimes be serious. Stop using NEMLUVIO and tell your healthcare provider or get emergency help right away if you get any of the following symptoms:
o breathing problems
or wheezingo swelling of the face, lips, mouth, tongue, or throat o fainting, dizziness, feeling lightheaded o fast pulse
o swollen lymph nodes o joint pain o fever o skin rash (red or rough skin) o nausea or vomiting o general ill feeling o cramps in your stomach area The most common side effects of NEMLUVIO in people treated for prurigo nodularis include:
- headache
- skin rashes: atopic dermatitis (a type of eczema), eczema, and eczema nummular (scattered circular patches)
The most common side effects of NEMLUVIO in people treated for atopic dermatitis include:
- headache
- joint pain
- hives (itchy red rash or wheals)
- muscle aches
These are not all of the possible side effects of NEMLUVIO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store NEMLUVIO?
- Store NEMLUVIO in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date.
- Store NEMLUVIO in the original carton to protect from light.
- NEMLUVIO can be stored at room temperature up to 77ºF (25ºC) for a single period up to 90 days.
- Throw away (dispose of) NEMLUVIO after the expiration date and any NEMLUVIO that has been left at room temperature for longer than 90 days.
- After the NEMLUVIO lyophilized powder and water for injection are mixed (reconstituted), NEMLUVIO must be used within 4 hours or thrown away (discarded).
- Do not heat or put NEMLUVIO pen into direct sunlight.
- Do not freeze the NEMLUVIO pen.
- If the NEMLUVIO pen was heated or frozen, throw it away (dispose of it).
Keep NEMLUVIO and all medicines out of the reach of children.
General information about the safe and effective use of NEMLUVIO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NEMLUVIO for a condition for which it was not prescribed. Do not give NEMLUVIO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NEMLUVIO that is written for healthcare professionals.What are the ingredients in NEMLUVIO?
Active ingredient: nemolizumab-ilto
Inactive ingredients: arginine hydrochloride, poloxamer 188, sucrose, trometamol, and tris hydrochloride.Manufactured by: Galderma Laboratories, L.P., Dallas, TX 75201
U.S. License No. 2289
NEMLUVIO® is a registered trademark of Galderma.
© 2025 Galderma Laboratories, L.P. All rights reserved.
For more information, go to www.NEMLUVIO.com or call 1-866-735-4137.This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 06/2025
-
INSTRUCTIONS FOR USE
NEMLUVIO® [Nem LOO vee oh]
(nemolizumab-ilto)
for injection, for subcutaneous use
This Instructions for Use contains information on how to inject NEMLUVIO.
Read and understand these instructions before using the NEMLUVIO pen.
Do not inject yourself or someone else until you have been instructed how to inject NEMLUVIO.
In adolescents (ages 12 to 17 years old), it is recommended that NEMLUVIO be given by or under supervision of a trained adult or caregiver.
Your healthcare provider will instruct you or your caregiver how to prepare and inject a dose of NEMLUVIO before you try to do it yourself the first time. Call your healthcare provider if you have any questions.
NEMLUVIO is supplied as a single-dose pen (called NEMLUVIO pen or pen in these instructions). It contains medicine (30 mg of lyophilized powder) in one chamber and water for dissolving the medicine in the other chamber. Before you can inject it, you must mix the lyophilized powder with the water for dissolving the medicine.
Important Information
What you need to know before using the NEMLUVIO pen:- Read all the instructions carefully before using the NEMLUVIO pen.
- Mark your calendar ahead of time to remember when to take NEMLUVIO.
- Do not use the NEMLUVIO pen if it has been dropped on a hard surface or is damaged, cracked or broken.
- Follow all steps exactly as described. This makes sure that you get the correct dose of medicine.
- Make sure that the lyophilized powder is completely dissolved before injecting (Step 9). After dissolving the powder, inject the medicine right away to avoid any contamination or break down of medicine (degradation).
- To use the pen for injection, while holding upright, gently twist (do not pull) the gray cap to the left (counterclockwise) until the orange needle guard pops up. Then, gently pull the gray cap off the orange needle guard. Do not press the orange needle guard until ready to inject (Step 12).
- In some cases, your healthcare provider may prescribe 2 pens for a full dose. Use the second NEMLUVIO pen right away after using the first pen. Choose a different injection site at least 1 inch away from the first injection site.
- To reduce the risk of accidental needle stick injury, each NEMLUVIO pen has an orange needle guard. After injecting the medicine and lifting the pen from your skin, the orange needle guard locks into place automatically to cover the needle (Step 16).
- Throw away (dispose of) the used NEMLUVIO pen right away after use in a sharps disposal container. See Section C: Throwing away (Disposing of) NEMLUVIO below.
Storage Information:
- Store the NEMLUVIO pen in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date.
- Store the NEMLUVIO pen in the original carton to protect it from light.
- The NEMLUVIO pen can be stored at room temperature up to 77°F (25°C) for up to 90 days.
- Throw away (dispose of) the NEMLUVIO pen after the expiration date and any NEMLUVIO pen that has been left at room temperature longer than 90 days.
- After the NEMLUVIO pen lyophilized powder and water for injection are mixed (reconstituted), the NEMLUVIO pen must be used within 4 hours or thrown away (disposed of).
- Do not heat or put the NEMLUVIO pen into direct sunlight.
- Do not freeze the NEMLUVIO pen.
- If the NEMLUVIO pen was heated or frozen, throw it away (dispose of it).
Keep the NEMLUVIO pen and all medicines out of the reach of children.
Traveling Information:
- Generally, you are allowed to carry pens with you on an airplane. Be sure to carry the NEMLUVIO pens in the original carton with you in your carry-on luggage.
If you have any other questions refer to the Frequently asked questions (FAQs) on the back of this leaflet.
A. Preparing to inject NEMLUVIO
Step 1: Let NEMLUVIO reach room temperature
Injecting cold medicine might result in pain at the injection site.
Take the NEMLUVIO carton out of the refrigerator and let it come to room temperature for 30 to 45 minutes before starting Step 2 (see Figure A).

Do not:
- warm the pen with any heat source (such as microwave or direct sunlight). This might damage NEMLUVIO.
- directly expose the pen to liquids.
Step 2: Wash your hands
a. Wash your hands with soap and water to avoid contamination and infection (see Figure B).
b. Dry hands completely.

Step 3: Gather Supplies (see Figure C)
a. Remove the pen from the carton.
b. Gather the following supplies on a clean, flat and well-lit surface:

- Pen
- Alcohol wipes*
- Sharps disposal container*
- Gauze pads or cotton balls*
*Items not included in the carton.
Note: In some cases, your healthcare provider may prescribe 2 pens. Use 1 pen after the other.
Step 4: Check the NEMLUVIO pen for the following:
a. Expiration date has not passed.
b. The lyophilized powder is white and not dissolved (see Figure D).
c. The pen has not been dropped and is not damaged or cracked.
Do not use the pen, unless all conditions above are met.
If any condition is not met, call: 1-866-735-4137.
Throw away (dispose of) the pen and use a new one (see Section C: Throwing away (Disposing of) NEMLUVIO).
Step 5: Activate the NEMLUVIO pen
Hold the pen upright and unlock the pen by turning the activation knob to the right until it stops (see Figure E).
This starts the process of transferring water to the powder chamber.
Only turn the activation knob to the right once.
Step 6: Wait until the gray rod stops moving
Watch inspection window until gray rod has stopped moving (see Figure F).
Do not shake the pen. Shaking the pen before the gray rod has completely stopped can affect the medicine dose.
Step 7: Dissolve the medicine
When the gray rod has completely stopped, hold the pen upright and shake the pen up and down for 30 seconds (see Figure G).
Step 8: Wait 5 minutes for bubbles to decrease
Wait for bubbles to decrease and the lyophilized powder to dissolve completely. This will take about 5 minutes (see Figure H).

Note: If the medicine has not dissolved completely, shake the pen up and down again for 30 seconds and then wait 5 minutes.
Note: It is normal for a small foam layer or a few small air bubbles to remain in the dissolved medicine.
Step 9: Check the medicine in the inspection window
Check to see if the dissolved medicine:
- is clear and colorless to slightly yellow,
- does not contain particles (see Figure I).

Do not use the pen if the dissolved medicine is cloudy or contains any particles.
Throw away (dispose of) the pen and use a new one (see Section C: Throwing away (Disposing of) NEMLUVIO).
Note: After the medicine has dissolved, it must be used within 4 hours. During this time, it should be kept at room temperature (up to 77°F (25°C)). If you have not used it within 4 hours, throw it away (dispose of it).B. Injecting NEMLUVIO
Step 10: Select one injection site (see Figure J)
Note: When using a second pen, select a different injection site at least 1 inch away from the first injection site.
Select the injection site using the following chart:
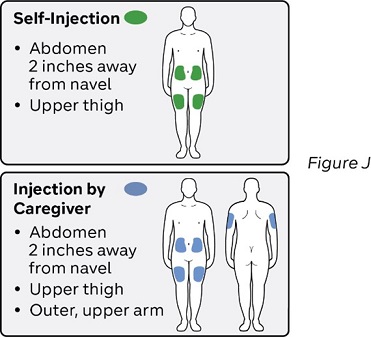
Where not to inject:
- near your waistline within about 2 inches around the navel.
- into tender, bruised, red skin, or areas with scars or stretch marks.
- twice into the same site (for example, within 1 inch).
Step 11: Clean the injection site (see Figure K)
a. Always use a new alcohol wipe to clean the injection site to avoid contamination and infection.
b. Let the skin air dry.

Do not:
- touch the injection site after cleaning.
- fan, or blow air on the cleaned injection site.
- reuse the alcohol wipe.
Step 12: Twist the gray cap
Do not:- pull the gray cap when twisting to avoid damaging the device.
- touch the orange needle guard.
- Hold the pen upright (see Figure L, a)
- Gently twist the gray cap to the left (counterclockwise) until the orange needle guard pops up (see Figure L, b).
- Gently pull the gray cap off the orange needle guard (see Figure L, c). Do not press on the orange needle guard until ready to inject.
- After cap removal, please throw away (dispose of) the cap in a sharps disposal container (see Step 17).
Note: If the cap cannot be removed, refer back to Step 5 and make sure the activation knob is turned completely to the right until it stops.

Step 13: Place the NEMLUVIO pen
Read Steps 13-16 before starting Step 13.
Note: Always inject the way your healthcare provider showed you.
Place the pen on the injection site vertically so that the orange needle guard is flat against the skin (see Figure M).
Note: Make sure you can easily see the inspection window during injection.

Step 14: Start injection and hold the NEMLUVIO pen on the skin
Gently push the pen down until the orange needle guard is completely pushed in.
The injection starts right away with a click (see Figure N).
The orange rod will begin to move down the injection window.
Do not lift the pen yet and keep pushing down.

Step 15: Inject for 15 seconds
Hold the pen in position and slowly count to 15 or use a timer or clock to count 15 seconds.
Check the inspection window to make sure the orange rod and gray rod have stopped moving (see Figure O).
This means the injection has been completed.
Note: It is normal that the orange rod does not cover the whole inspection window at the end of injection.

Do not lift the pen until the orange rod and gray rod have stopped moving.
If the orange rod is not visible, please call: 1-866-735-4137. Throw away (dispose of) the pen and use a new one (see Section C for disposal details).
Step 16: Lift the NEMLUVIO pen up
a. Lift the pen straight up from your skin.
The orange needle guard automatically locks into place to cover the needle (see Figure P).
b. If there is bleeding, press a cotton ball or gauze over the injection site.

Do not rub the injection site.

C. Throwing away (disposing of) NEMLUVIO
Step 17: Throw away (dispose of) NEMLUVIO in a sharps disposal container
Avoid contact with the needle.
Throw away (dispose of) the used pen and the gray cap in an FDA cleared sharps disposal container right away after use (see Figure Q).

Do not:
- recap the pen after use,
- dispose of the NEMLUVIO pen and cap in your household trash,
- dispose of your used sharps disposal container in your household trash unless your community guidelines permit this,
- recycle your used sharps disposal container.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should dispose of used pens.For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal
FAQs (Frequently asked questions)
Needle Injecting the medicine Where is the needle? Why do I need to hold the pen upright while removing the gray cap? The needle is attached to the NEMLUVIO pen and covered by the gray cap.
When you twist the gray cap, the orange needle guard pops up and covers the needle until you inject the medicine.
For more information, please see the figures in Step 12 in this Instructions for Use.Holding the NEMLUVIO pen with the gray cap upright helps prevent the medicine from leaking. It is normal to see a few drops of medicine inside the gray cap even when you hold the pen upright and remove the gray cap. Dissolving the medicine How do I know I injected myself the full dose of medicine in the pen? How do I know if the medicine is fully dissolved?
To be sure you get the full dose of medicine, press and hold the NEMLUVIO pen against your skin.
You will feel the needle go into your skin. Hold the NEMLUVIO pen against your skin for 15 seconds. This will allow enough time for all the medicine to go from the pen to under your skin.
Only remove the NEMLUVIO pen when the orange rod and the gray rod have stopped moving.
After removing the NEMLUVIO pen, look for the orange rod in the window as a way to tell that the dose has been given. If the orange rod does not appear, contact 1-866-735-4137.
For details please refer to Step 15 in this Instructions for Use.To dissolve, shake the NEMLUVIO pen in an upright position, up and down, until the white particles are no longer on the bottom, top, or sides. The dissolved medicine should look clear. Please refer to Step 9 in this Instructions for Use.
After shaking the NEMLUVIO pen, look through both sides of the inspection window. You should not see any white particles along the bottom, top, or sides.
It is acceptable to have small air bubbles or a small foam layer on top of the medicine. It does not harm you.
If you see white particles in the medicine, the medicine powder is not fully dissolved.
Storage General Information
How should I store the NEMLUVIO pen? Is it necessary to receive instructions on how to use the NEMLUVIO pen from a healthcare provider?
The NEMLUVIO pen should be stored in the refrigerator in its original carton to protect it from light. It can be stored at room temperature for up to 77°F (25°C) for a single period up to 90 days.
The device should not come in contact with liquids.Yes.
Do not inject the medicine if you did not receive a demonstration by your healthcare provider.
You should contact your healthcare provider right away to receive the information and instructions on how to use the NEMLUVIO pen.Where do I find the expiration date? Where can I get more information about using NEMLUVIO: You can find the expiration date on the NEMLUVIO pen labeled as
EXP YYYY-MM.
Do not use the NEMLUVIO pen past the expiration date.If you have other questions about how to use the NEMLUVIO pen:
- Call your healthcare provider
- Call 1-866-735-4137What should I do if the medicine has been frozen? Do not use the NEMLUVIO pen if it has been frozen. Throw away (dispose of) the NEMLUVIO pen and use a new one. Manufactured by:
Galderma Laboratories, L.P.
Dallas, TX 75201
This Instructions for Use has been approved by the U.S. Food and Drug Administration
U.S. License No. 2289
Issued: 06/2025
-
PRINCIPAL DISPLAY PANEL 30 mg PRE-FILLED DUAL-CHAMBER PEN CARTON
IMPORTANT:
CAREFULLY read and follow Instructions for Use.
This pen requires specific steps before injection.
1 Single-Dose Pre-Filled Dual-Chamber Pen
Keep out of reach of children. Do NOT use after expiration.
Do NOT use if seal is broken or damaged. Dosage: See Prescribing
For Subcutaneous Use
MUST BE RECONSTITUTED BEFORE INJECTION
Single-Dose only
GALDERMA
NDC: 0299-6220-15 Rx only
nemluvio™
(nemluvio-ilto) for injection 30 mg -
INGREDIENTS AND APPEARANCE
NEMLUVIO
nemolizumab-ilto injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0299-6220 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nemolizumab (UNII: GN465U8B72) (Nemolizumab - UNII:GN465U8B72) Nemolizumab 30 mg in 100 mg Inactive Ingredients Ingredient Name Strength Sucrose (UNII: C151H8M554) Tromethamine (UNII: 023C2WHX2V) Tromethamine Hydrochloride (UNII: 383V75M34E) Poloxamer 188 (UNII: LQA7B6G8JG) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0299-6220-15 1 in 1 CARTON 08/13/2024 1 30 mg in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0299-6220-10 1 in 1 CARTON 08/13/2024 2 30 mg in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761390 08/13/2024 Labeler - Galderma Laboratories, L.P. (047350186) Registrant - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Mooswiesen) 312670654 manufacture(0299-6220) Establishment Name Address ID/FEI Business Operations SHL Medical Assembly & Services LLC 119581224 label(0299-6220) , pack(0299-6220)
Trademark Results [NEMLUVIO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEMLUVIO 79387842 not registered Live/Pending |
Galderma Holding SA 2023-12-01 |
 NEMLUVIO 79382487 not registered Live/Pending |
Galderma Holding SA 2023-08-11 |
 NEMLUVIO 79340482 not registered Live/Pending |
Galderma Holding SA 2022-02-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.