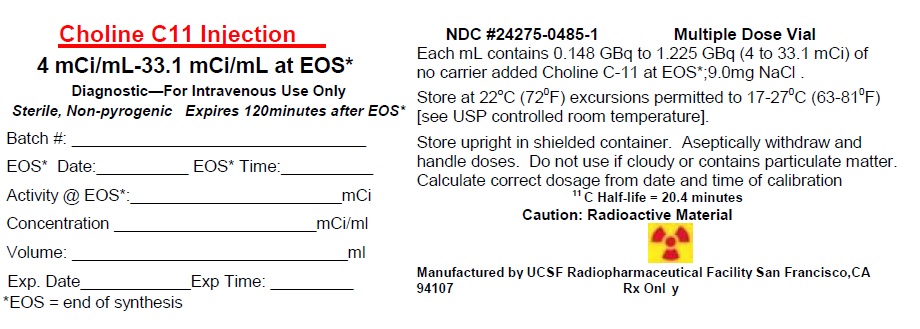
CHOLINE C 11- choline c-11 injection
Choline C 11 by
Drug Labeling and Warnings
Choline C 11 by is a Prescription medication manufactured, distributed, or labeled by UCSF Radiopharmaceutical Facility. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CHOLINE C 11 INJECTION safely and effectively. See full prescribing information for CHOLINE C 11 INJECTION.
CholineC 11 Injection, for intravenous use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
Choline C 11 Injection is a radioactive diagnostic agent for positron emission tomography (PET) imaging of patients with suspected prostate cancer recurrence and non-informative bone scintigraphy, computerized tomography (CT) or magnetic resonance imaging. In these patients, 11 C-choline PET imaging may help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation. Suspected prostate recurrence is based upon elevated blood prostate specific antigen (PSA) levels following initial therapy. In clinical studies, images were produced with PET/CT coregistration.
Limitation of Use:11 C-choline PET imaging is not a replacement for histologic verification of recurrent prostate cancer (1).DOSAGE AND ADMINISTRATION
- Aseptically withdraw Choline C 11 Injection from its container and administer 370 – 740 MBq (10 – 20 mCi) as a bolus intravenous injection. The radioactivity dose (370 – 740 MBq, 10 – 20 mCi) is chosen based on patient body dimensions and the characteristics of the image acquisition system (2.1).
- Initiate imaging immediately after administration of Choline C 11 Injection and acquire static emission images 0 – 15 minutes from the time of injection (2.5).
- The effective radiation absorbed dose from 740 MBq (20 mCi) dose of Choline C 11 Injection is approximately 3.22 mSv (0.32 rem) in an adult (2.4).
- Image interpretation: Refer to full prescribing information (2.5).
DOSAGE FORMS AND STRENGTHS
- Choline C 11 Injection contains 148 – 1,225 MBq (4 – 33.1 mCi) per milliliter of 11C-choline at end of synthesis calibration time in aqueous 0.9% sodium chloride solution (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- Imaging errors have been reported; blood PSA levels < 2 ng/mL have been associated with poor imaging performance (5.1).
- Allergic reactions: have emergency resuscitation equipment and personnel readily available (5.2).
- Radiation risk: Choline C 11 Injection contributes to a patient’s long-term cumulative radiation exposure. Ensure safe handling to protect the patient and health care worker (5.3).
ADVERSE REACTIONS
Exclusive of an uncommon, mild injection site reaction, no other adverse reactions have been reported (6).
To report SUSPECTED ADVERSE REACTIONS, contact UCSF Radiopharmaceutical Facility at 415-353-4435 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
Colchicine and androgen-deprivation therapeutic drugs may interfere with 11C-choline PET/CT imaging performance (5.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2016
- Aseptically withdraw Choline C 11 Injection from its container and administer 370 – 740 MBq (10 – 20 mCi) as a bolus intravenous injection. The radioactivity dose (370 – 740 MBq, 10 – 20 mCi) is chosen based on patient body dimensions and the characteristics of the image acquisition system (2.1).
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1Radiation Safety - Drug Handling
2.2Recommended Dose and Administration Instructions
2.3Patient Preparation
2.4Radiation Dosimetry
2.5Imaging Guidelines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1Imaging Errors
5.2Allergic Reactions
5.3Radiation Risks
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3Nursing Mothers
8.4Pediatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2Physical Characteristics
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2Pharmacodynamics
12.3Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1INDICATIONS AND USAGE
Choline C 11 Injection is indicated for positron emission tomography (PET) imaging of patients with suspected prostate cancer recurrence and non-informative bone scintigraphy, computerized tomography (CT) or magnetic resonance imaging (MRI). In these patients, 11 C-choline PET imaging may help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation. Suspected prostate recurrence is based upon elevated blood prostate specific antigen (PSA) levels following initial therapy. In clinical studies, images were produced with PET/CT coregistration.
Limitation of Use:11 C-choline PET imaging is not a replacement for histologic verification of recurrent prostate cancer.
-
2 DOSAGE AND ADMINISTRATION
2.1Radiation Safety - Drug Handling
Choline C 11 Injection is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration. Use waterproof gloves and effective shielding when handling Choline C 11 Injection. Radiopharmaceuticals, including Choline C 11 Injection, should only be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radioactive materials, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
2.2Recommended Dose and Administration Instructions
The recommended dose is 370 – 740 MBq (10 – 20 mCi) administered as a bolus intravenous injection. The radioactivity dose (370 – 740 MBq, 10 – 20 mCi) is chosen based on patient body dimensions and the characteristics of the image acquisition system
- Inspect Choline C 11 Injection visually for particulate matter and discoloration before administration. Do not use the drug if the solution contains particulate matter or is discolored.
- Aseptically withdraw Choline C 11 Injection from its container and administer the drug as a bolus through a peripheral venous catheter.
- Dispose of any unused drug in a safe manner, in compliance with applicable regulations.
2.3Patient Preparation
Prior to administration of Choline C 11 Injection:
- Fasting for at least six hours is recommended to minimize the potential for dietary choline interference with radioactivity uptake in tissue.
- Ensure that the patient is well hydrated and encourage voiding when imaging is completed.
2.4Radiation Dosimetry
The estimated radiation absorbed doses for adults from intravenous injection of Choline C 11 Injection are shown in Table 1. These estimates are calculated from data in Tolvanen 1and using OLINDA/EXM (Organ Level Internal Dose Assessment/Exponential Modeling) software from Vanderbilt University.2
Table 1: Estimated Radiation Absorbed Dose Per Unit Activity for Adults, Choline C 11 Injection Organ/Tissue Mean Absorbed Dose Per Unit Administered Activity (μGy/MBq)b Adrenals 3.59 Bone – Osteogenic Cells 4.81 Bone – Red Marrow 1.90 Brain 1.16 Breast 1.39 Gallbladder Wall 4.54 GIa – Lower Large Intestine Wall 1.81 GIa – Small Intestine 2.35 GIa – Stomach Wall 6.00 GIa – Upper Large Intestine Wall 6.41 Heart wall 3.43 Kidneys 20.62 Liver 20.11 Lungs 4.59 Muscle 2.54 Ovaries 2.02 Pancreas 29.19 Skin 1.22 Spleen 9.16 Testes 1.36 Thymus 1.69 Thyroid 1.49 Urinary Bladder Wall 3.41 Uterus 1.96 Total body 2.97 Effective Dose (μSv/MBq)c 4.35 a Gastrointestinal
b Assumed radiation weighting factor, wr, (formerly defined as quality factor, Q) of 1 for conversion of absorbed dose (Gray or rad) to dose equivalent (Sieverts or rem) for C 11. To obtain radiation absorbed dose in rad/mCi from the above table, multiply the dose in μGy/MBq by 0.0037, (e.g., 3.59 μGy/MBq × 0.0037 = 0.0133 rad/mCi).
c Radiation tissue weighting factors, wT, used in the calculation of effective dose are from 1990 Recommendations of the International Commission on Radiological Protection, ICRP Publication 60 (1991). To obtain radiation absorbed dose in rem/mCi from above table, multiply the dose in μGy/MBq by 0.0037, (e.g., 4.35 μGy/MBq × 0.0037 = 0.0161 rem/mCi).The effective dose resulting from a 740 MBq (20 mCi) dosage of Choline C 11 Injection is 3.22 mSv in an adult, (740 × 4.35 = 3219 μSv = 3.2 mSv). The use of a CT scan to calculate attenuation correction for reconstruction of 11C-choline images (as done in PET/CT imaging) will add radiation exposure. Based upon current scanning techniques, an effective dose of 5.8 mSv would be added from CT scanning. The actual radiation dose is operator, scanner, and patient dependent. The total radiation exposure from 11C-choline administration and subsequent scan on a PET/CT scanner is estimated to be 9.0 mSv (3.2 mSv + 5.8 mSv).
2.5Imaging Guidelines
- Initiate image acquisition immediately after administration of Choline C 11 Injection. Imaging is typically performed from the base of the pelvis to the base of the skull.
- Acquire static emission images 0 – 15 minutes from the time of injection.
- Localized uptake of 11C-choline in a site suspicious for prostate cancer recurrence (a positive image) is determined by comparison of the anatomical relationship of concentrated radioactivity to the neighboring tissue background, exclusive of the radioactivity physiologically accumulated within the pancreas, liver, spleen, kidney and colon.
- Inspect Choline C 11 Injection visually for particulate matter and discoloration before administration. Do not use the drug if the solution contains particulate matter or is discolored.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1Imaging Errors
Imaging errors have been reported with 11C-choline PET and PET/CT imaging. A negative image does not rule out the presence of recurrent prostate cancer and a positive image does not confirm the presence of recurrent cancer. 11C-choline uptake is not specific for prostate cancer and may occur with other types of cancer (such as lung carcinoma and brain tumors). Clinical correlation, including histopathological evaluation of the suspected recurrence site, is essential to proper use of the PET imaging information.
- Blood PSA levels < 2 ng/mL have been associated with poor performance of 11C-choline PET imaging (higher numbers of false positive and false negative results) [see Clinical Studies (14)].
- Tissue inflammation as well as prostatic hyperplasia have been associated with false positive 11C-choline PET images.
- Concomitant colchicine or androgen-deprivation therapeutic drugs (such as luteinizing hormone-releasing analogs and anti-androgen drugs) may interfere with 11C-choline PET imaging. One published report of 18F-methylcholine PET imaging indicated that discontinuation of colchicine for two weeks resolved the colchicine effect. The impact of discontinuation of androgen-deprivation therapy upon 11C-choline PET imaging has not been established [see Drug Interactions(7)].
5.2Allergic Reactions
As with any injectable drug product, allergic reactions and anaphylaxis may occur. Emergency resuscitation equipment and personnel should be immediately available.
5.3Radiation Risks
Choline C 11 Injection contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Safe handling should be ensured to minimize radiation exposure to the patient and health care workers [seeDosage and Administration (2.1)].
- Blood PSA levels < 2 ng/mL have been associated with poor performance of 11C-choline PET imaging (higher numbers of false positive and false negative results) [see Clinical Studies (14)].
- 6 ADVERSE REACTIONS
-
7 DRUG INTERACTIONS
Colchicine and androgen-deprivation therapeutic drugs have been reported to interfere with choline-based PET imaging [see Warnings and Precautions (5.1)].
The impact of androgen-deprivation therapeutic drugs upon 11C-choline PET imaging may depend upon the hormonal responsiveness of a patient’s recurrent prostate cancer. Clinical studies have not established this relationship but published reports suggest 11C-choline PET imaging may be productive in patients with “hormone resistant” recurrent prostate cancer even if the patients are receiving anti-androgen therapy. Imaging may prove unproductive or misleading due to failed or insufficient 11C-choline uptake in patients with hormone-responsive cancer if the patients are receiving androgen-deprivation therapy.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well controlled studies with Choline C 11 Injection in pregnant women and the fetal radiation dose from a 11C-choline PET imaging study is unknown. It is not known whether Choline C 11 Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Animal reproduction studies have not been conducted with 11C-choline.All radiopharmaceuticals, including Choline C 11 Injection, have a potential to cause fetal harm. The likelihood of fetal harm depends on the stage of fetal development and the magnitude of the radiopharmaceutical dose. Assess pregnancy status before administering Choline C 11 Injection to a female of child bearing potential. Choline C 11 Injection should be given to a pregnant woman only if clearly needed.
8.3Nursing Mothers
Choline C 11 Injection is not indicated for use in women. It is not known whether Choline C 11 Injection is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for radiation exposure to nursing infants from Choline C 11 Injection, nursing mothers should use alternative infant nutrition sources (e.g., stored breast milk or infant formula) and pump and discard breast milk for 8 hours (>10 half lives of radioactive decay for 11C isotope) after administration of the drug or avoid use of the drug, taking into account the importance of the drug to the mother.
-
11 DESCRIPTION
11.1 Chemical Characteristics
Choline C 11 Injection is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with PET imaging. The active ingredient, 11C-choline, has the molecular formula of C411CH14 NOCl with a molecular weight of 138.63 g and has the following chemical structure:
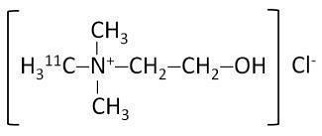
Choline C 11 Injection is provided as a ready to use sterile, pyrogen-free, clear and colorless solution. Each milliliter contains 148 – 1,225 MBq (4 – 33.1 mCi) of 11C-choline at EOS calibration time in aqueous 0.9% sodium chloride solution. The pH of the solution is between 4.5 and 7.5.
11.2Physical Characteristics
Carbon 11 is a cyclotron-produced radionuclide that decays to Boron 11 by positron emission and has a physical half life of 20.4 minutes (Table 2).
Table 2: Principal Radiation Emission Data for 11C Radiation/Emission % Per Disintegration Energy Positron (β+)
99.76 960.2 keV (Max.) Gamma (±)*
199.5 511 keV *Produced by positron annihilation
The specific gamma ray constant (point source air kerma coefficient) for 11C-choline is 5.8 R/mCi-hr at 1 cm. Selected coefficients of attenuation are listed in Table 3 as a function of lead shield thickness. For example, the use of 39 mm thickness of lead will attenuate the external radiation by a factor of about 1,000.
Table 3: Radiation Attenuation of 511 keV Photons by lead (Pb) shielding Shield Thickness (Pb) mm Coefficient of Attenuation 4 0.5 8 0.25 13 0.1 26 0.01 39 0.001 52 0.0001 Table 4 lists fractions remaining at selected time intervals from the calibration time.This information may be used to correct for physical decay of the radionuclide.
Table 4: Physical Decay Chart for 11C Minutes Fraction Remaining 0* 1.000 5 0.844 10 0.712 15 0.600 20 0.507 25 0.427 30 0.360 *Calibration time
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Choline C 11 Injection is a radiolabeled analog of choline, a precursor molecule essential for the biosynthesis of cell membrane phospholipids. Choline is involved in synthesis of the structural components of cell membranes, as well as modulation of trans-membrane signaling. Increased phospholipid synthesis (i.e., increased uptake of choline) has been associated with cell proliferation and the transformation process that occurs in tumor cells.
12.2Pharmacodynamics
In a study of men with prostatic hyperplasia or primary prostate cancer, PET imaging showed 11C-choline radioactivity accumulated rapidly within the prostate; uptake appeared to peak by five minutes following injection of the drug and activity was retained over the subsequent 30 minute scanning period. Little uptake was observed in the bladder and rectum.
12.3Pharmacokinetics
Distribution: 11C-choline distributes mainly to the pancreas, kidneys, liver, spleen and colon [see Dosage and Administration (2.4)]. Based upon the relatively low urinary excretion of radioactivity, renal distribution is predominantly to the organ itself, rather than via formation of urine.
Metabolism: Following intravenous administration, 11C-choline undergoes metabolism resulting in the detection of 11C-betaine as the major metabolite in blood. In a study of patients with prostate cancer or brain disorders, the fractional activities of 11C-choline and 11C-betaine in human arterial plasma appeared to reach a plateau within 25 minutes, with 11C-betaine representing 82± 9% of the total 11C detected at that time point. A small amount of unmetabolized 11C-choline was detected within the blood at the final sampling time point (40 minutes).
Elimination: Urinary excretion of 11C-choline was < 2% of the injected radioactivity at 1.5 hours after injection of the drug. The rate of 11C-choline excretion in urine was 0.014 mL/min.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies have not been performed to evaluate the carcinogenic potential of Choline C 11 Injection. The mutagenic potential of Choline C 11 Injection has not been adequately evaluated; however, any radiopharmaceutical, including Choline C 11 Injection, has the potential to be mutagenic. The effect of Choline C 11 Injection on fertility has not been evaluated.
-
14 CLINICAL STUDIES
A systematic review of published reports identified four studies that contained data sufficient to compare 11C-choline PET imaging to histopathology (truth standard) among patients with suspected prostate cancer recurrence and non-informative conventional imaging (for most patients, CT or MRI). In general, the suspected recurrence criteria consisted of at least two sequential PSA levels of > 0.2 ng/mL for men who had undergone prostatectomy and PSA levels of ≥ 2 ng/mL above the post-therapy nadir for men who had undergone radiotherapy. The studies were predominantly single clinical site experiences and image acquisition generally surveyed radioactivity distribution from the base of the pelvis to the base of the skull.
Prospective studies: Two studies examined the ability of 11C-choline PET/CT to detect prostate cancer in pelvic and/or retroperitoneal lymph nodes among patients who had previously undergone radical prostatectomy. Both studies used a truth standard of lymph node histopathology. 11C-choline images were interpreted by readers masked to clinical information; surgical resection of lymph nodes was performed by surgeons aware of the 11C-choline PET/CT results.
In Study One3, 25 patients who underwent 11C-choline PET/CT and conventional imaging (CT or MRI) were scheduled to undergo pelvic or pelvic plus retroperitoneal lymphadenectomy following the imaging identification of suspected lymph node metastases. The median PSA was 2.0 ng/mL (range 0.2 to 23.1 ng/mL). The study excluded subjects with metastatic disease detected by bone scintigraphy or isolated prostatic fossa recurrence. Among the 25 patients, 21 had positive 11C-choline PET/CT scans; histopathology verified cancer in 19 of these patients. Lymph node histopathology detected no cancer among the four patients who had surgery based only on positive conventional imaging; 11C-choline PET/CT was negative in all four patients. The study report included information for patients who had non-informative conventional imaging (CT or MRI, bone scintigraphy and transrectal ultrasound), as shown in Table 5.
In Study Two4, 15 patients were scheduled to undergo pelvic or pelvis plus retroperitoneal lymphadenectomy solely based upon positive 11C-choline PET/CT imaging in the setting of negative conventional imaging (ultrasound and/or CT and/or MRI and/or bone scintigraphy). The median PSA was 2.0 ng/mL (range 1.0 to 8.0 ng/mL); all patients had previously undergone radical prostatectomy. Eight of the 15 patients had cancer verified by lymph node histology; histology detected no cancer in seven patients.
Retrospective Studies: Two studies were retrospective reviews of patients who underwent 11C-choline PET/CT and had histopathology obtained from biopsy of the prostatic fossa or other suspected recurrence sites.
In Study Three5, 11C-choline PET/CT imaging was performed among 36 patients with suspected prostate cancer recurrence and 13 subjects without suspected recurrence (controls). Prostatic fossa biopsies were performed among the patients with suspected recurrence. All the patients and control subjects had previously undergone radical prostatectomy; patient with suspected recurrence had no evidence of cancer using conventional clinical evaluations, including trans-rectal ultrasound and bone scintigraphy. PET/CT scans were interpreted by readers masked to clinical information. Median PSA was 2.0 ng/mL (range 0.3 – 12.1 ng/mL) for patients with suspected recurrence and 0.1 ng/mL (range 0.0 – 0.2 ng/mL) in control subjects. Prostatic fossa biopsy showed cancer in 33 of the 36 patients with suspected recurrence. PET/CT scans were positive in 25 of the 36 patients; two patients had false positive scans (one scan in a control subject and one scan in a suspected recurrence subject who had no cancer detected on prostatic fossa biopsy). Among the 13 control subjects, 12 had negative PET/CT scans.
In Study Four6,7, 34 patients with negative conventional imaging underwent 11C-choline PET/CT and subsequently had biopsies of suspected recurrence sites. The median PSA level of the 34 patients was 3.9 ng/mL (range 0.2 – 65.0 ng/mL); 22 of the patients had previously undergone radical prostatectomy and 12 had received other therapy (radiotherapy, anti-androgen therapy or cryotherapy). 11C-choline PET/CT images were positive in 30 patients and negative in four patients. Cancer was verified by histopathology in 29 patients; 25 had positive PET/CT images and four had negative PET/CT images. Five patients with positive PET/CT images did not have cancer confirmed with histopathology.
As shown in Table 5, within each study at least half the patients with non-informative conventional imaging had positive 11C-choline PET/CT images and histologically verified recurrent prostate cancer.
Table 5 11 C-Choline PET/CT Results among Patients with Non-informative Conventional Imaging and a Histopathology Truth Standard Study Patients, n Total True Positive False Positive True Negative False Negative One 13 11 2 ND* ND* Two 15 8 7 ND* ND* Three 36 23 1 2 10 Four 34 25 5 0 4 *ND = not determined
In Studies Three and Four, PSA levels were generally lower for patients with negative 11 C-choline PET/CT results than for patients with positive results. In Study Three, the median PSA was 2.6 ng/mL (range 0.6 – 12.1 ng/mL) among the 23 patients with true positive images; nine out of 11 patients with false negative or false positive images had PSA levels < 2 ng/mL. In Study Four, the median PSA was 4.2 ng/mL (range 0.2 – 65.0 ng/mL) among the 25 patients with true positive images; PSA levels < 2 ng/mL were observed in four of the nine patients with false negative or false positive images. These data, combined with other published reports, suggest that 11C-choline PET imaging performance may be more reliable among patients with blood PSA levels > 2 ng/mL, compared to patients with lower levels.
-
15 REFERENCES
1Tolvanen T, Yli-Kerttula T, Ujula T, Autio A, Lehikoinen P, Minn J, RoivinenA; Biodistribution and radiation dosimetry of [11C] choline: a comparison between rat and human data. Eur J Nucl Med Mol Imaging. 2010; 37:874-83.
2OLINDA/EXM software, Version 1.1. Vanderbilt University, 2007.
3Scattoni V, Picchio M, Suardi N, Messa C, Freschi M, Roscigno M, Da Pozzo L, Bocciardi A, Rigatti P, Fazio F. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. EurUrol. 2007; 52:423-9.
4Rinnab L, Mottaghy FM, Simon J, Volkmer BG, de Petriconi R, Hautmann RE, Wittbrodt M, Egghart G, Moeller P, Blumstein N, Resks S, Kuefer R. [11C]choline PET/CT for targeted salvage lymph node dissection in patients with biochemical recurrence after primary curative therapy for prostate cancer. Urologia Int. 2008; 81:191-7.
5Reske SN, Blumstein NM, Glatting G. [11C]choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur J Med Mol Imaging. 2008; 35:9-17.
6Mitchell C, Kwon E, Lowe V, Hung J, Rangel L, Karnes RJ. Impact of 11C-choline PET/CT scan on detection of recurrent prostate cancer in men with biochemical recurrence following failed initial treatment; supplemented with subject-level data. J Urol. 2012; 187:e823.
7Mitchell C, Kwon E, Lowe V, Hung J, Rangel L, Karnes RJ. Detection of consolidated disease recurrences of prostate cancer by 11C-choline PET/Scan: results confirmed by surgical resection; supplemented with subject-level data. J Urol. 2012; 187:e823.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Instruct patients to drink plenty of water or other fluids (as tolerated) in the four hours before their PET/CT study.
- Instruct patients to void after completion of each image acquisition session and as often as possible for one hour after the PET/CT scan ends.
Manufactured by:
UCSF Radiopharmaceutical Facility
185 Berry Street Suite 350
San Francisco, California 94107Distributed by:
UCSF Radiopharmaceutical Facility
185 Berry Street Suite 350
San Francisco, Califronia 94107 - Instruct patients to drink plenty of water or other fluids (as tolerated) in the four hours before their PET/CT study.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHOLINE C 11
choline c-11 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24275-0485 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLINE C-11 (UNII: M4AS4XGD4Q) (CHOLINE C-11 - UNII:M4AS4XGD4Q) CHOLINE C-11 33.1 mCi in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24275-0485-1 1 in 1 PACKAGE 11/27/2017 1 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208444 11/27/2017 Labeler - UCSF Radiopharmaceutical Facility (831727388) Establishment Name Address ID/FEI Business Operations UCSF Radiopharmaceutical Facility 831727388 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(24275-0485)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.