Harry's Antiperspirant Wildlands
Harrys Antiperspirant Wildlands by
Drug Labeling and Warnings
Harrys Antiperspirant Wildlands by is a Otc medication manufactured, distributed, or labeled by Harry's, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
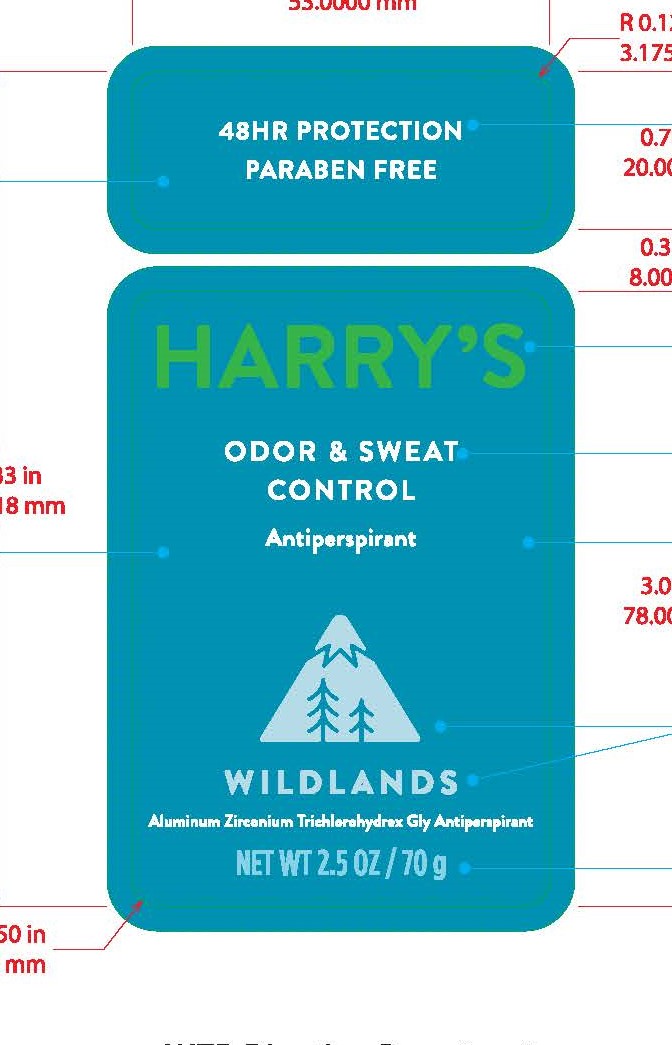
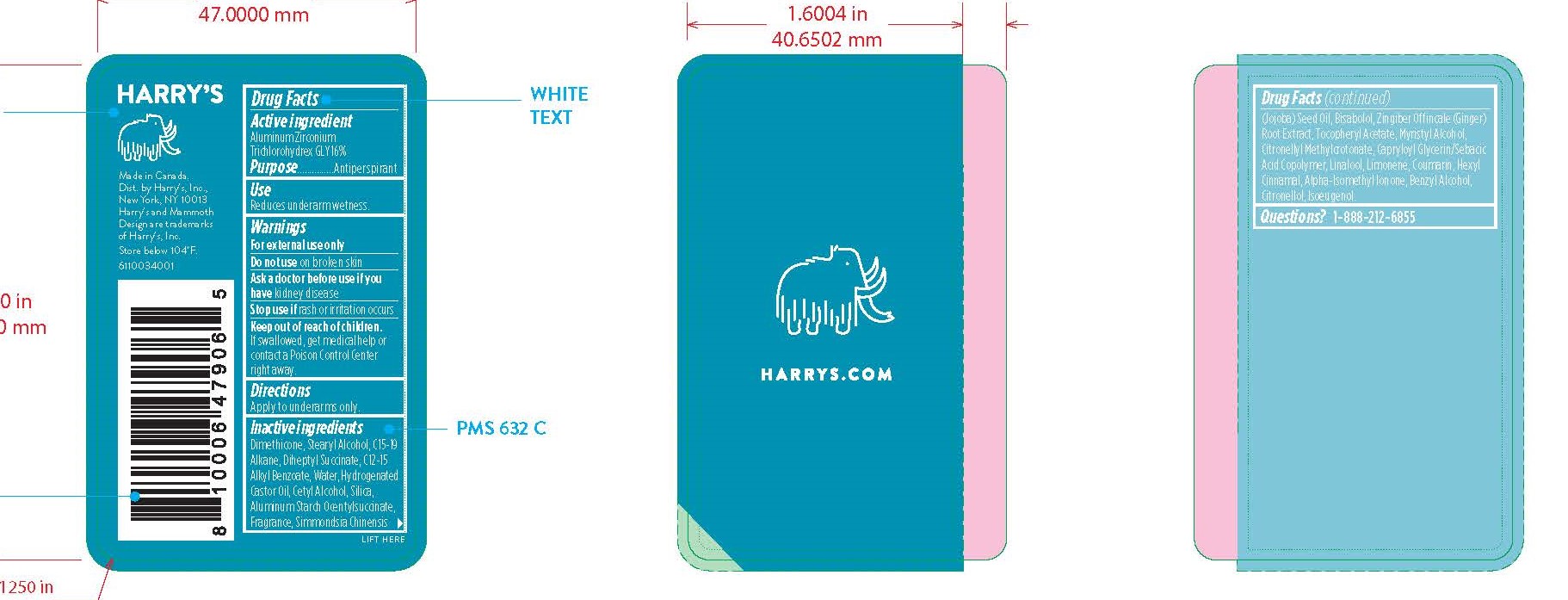
HARRYS ANTIPERSPIRANT WILDLANDS- aluminum zirconium trichlorohydrex gly stick
Harry's, Inc.
----------
Harry's Antiperspirant Wildlands
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
DIMETHICONE, STEARYL ALCOHOL, C15-19 ALKANE, DIHEPTYL SUCCINATE, C12-15 ALKYL BENZOATE, WATER, HYDROGENATED CASTOR OIL, CETYL ALCOHOL, SILICA, ALUMINUM STARCH OCENTYLSUCCINATE, FRAGRANCE, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, BISABOLOL, ZINGIBER OFFINCALE (GINGER) ROOT EXTRACT, TOCOPHERYL ACETATE, MYRISTYL ALCOHOL, CITRONELLYL METHYLCROTONATE, CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER, LINALOOL, LIMONENE, COUMARIN, HEXYL CINNAMAL, ALPHA-ISOMETHYL IONONE, BENZYL ALCOHOL, CITRONELLOL, ISOEUGENOL
| HARRYS ANTIPERSPIRANT WILDLANDS
aluminum zirconium trichlorohydrex gly stick |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Harry's, Inc. (079239206) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.