MAGNELEAF WITH CBD by M J Green Enterprises, Inc. / M. J Green Enterprises, Inc.
MAGNELEAF WITH CBD by
Drug Labeling and Warnings
MAGNELEAF WITH CBD by is a Homeopathic medication manufactured, distributed, or labeled by M J Green Enterprises, Inc., M. J Green Enterprises, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
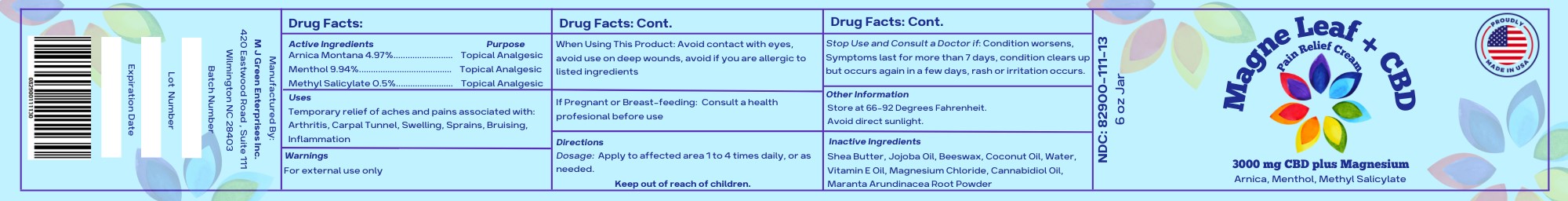
MAGNELEAF WITH CBD- menthol, arnica montana, methyl salicylate cream
M J Green Enterprises, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Uses
Temporary relief of aches and pains associated with: Arthritis, Carpal Tunnel, Swelling, Sprains, Bruising, Inflammation
When Using this Product:
Avoid contact with eyes, avoid use on deep wounds, avoid if you are allergic to listed ingredients.
| MAGNELEAF WITH CBD
menthol, arnica montana, methyl salicylate cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - M J Green Enterprises, Inc. (099497973) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| M. J Green Enterprises, Inc. | 099497973 | manufacture(82900-111) | |
Revised: 9/2023
Document Id: 05a44668-d4b8-b5b8-e063-6294a90a355c
Set id: e9e86ab0-12a6-58fb-e053-2995a90a5916
Version: 2
Effective Time: 20230918
 - 6 oz jar
- 6 oz jar
