GALLIUM- gallium citrate ga-67 injection
Gallium by
Drug Labeling and Warnings
Gallium by is a Prescription medication manufactured, distributed, or labeled by Lantheus Medical Imaging, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Gallium Citrate Ga 67 Injection is supplied in isotonic solution as a sterile, non-pyrogenic diagnostic radiopharmaceutical for intravenous administration. Each milliliter of the isotonic solution contains 74 MBq (2 mCi) of Gallium Ga 67 on the calibration date, as a complex formed from 9 ng Gallium Chloride Ga 67, 2 mg of sodium citrate, 6.8 mg sodium chloride, and 9 mg benzyl alcohol/mL added as preservative. The pH is adjusted to between 4.5-8 with hydrochloric acid and/or sodium hydroxide solution. Gallium Ga 67, with a half-life of 78.3 hours, is cyclotron produced by the proton irradiation of enriched zinc oxide, is essentially carrier-free and contains negligible concentrations of other radioactive isotopes.
The radionuclidic composition at calibration time is ≥99.89% Gallium Ga 67, ≤0.01% Gallium Ga 66 and ≤0.1% due to other radiocontaminants, each expressed as a percentage of total activity. The radionuclidic composition at expiration time is ≥99.89% Gallium Ga 67, essentially zero (0.0002%) Gallium Ga 66 and essentially zero of other radiocontaminants each expressed as a percentage of total activity.
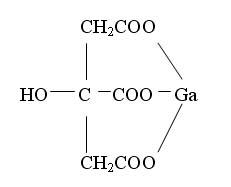
The chemical structure for Gallium Citrate is shown below:
Physical Characteristics
Gallium Ga 67 decays to stable Zinc Zn 67 by electron capture with a physical half-life of 78.3 hours.1
TABLE 1. Principal Radiation Emission Data Radiation Mean %/Disintegration Mean Energy (keV) Gamma-3 35.7 93.3 Gamma-4 19.7 184.6 Gamma-6 16.0 300.2
- 1 Kocher, David C., "Radioactive Decay Data Tables", DOE/TIC-11026 (1981).
External Radiation
The specific gamma ray constant for Gallium Ga 67 is 5.58 microcoulombs/Kg-hr-MBq (0.80R/hr-mCi) at 1 cm. The first half value thickness of lead is 0.066 cm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from the interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.41 cm of Pb will decrease the external radiation exposure by a factor of 10.
TABLE 2. Radiation Attenuation By Lead Shielding cm of Pb Radiation
Attenuation Factorcm of Lead
( Pb)Radiation
Attenuation Factor0.066 0.5 2.5 10-3 0.41 10-1 4.8 10-4 1.2 10-2 To correct for physical decay of this radionuclide, the fractions that remain at selected time intervals after the time of calibration are shown in Table 3.
-
CLINICAL PHARMACOLOGY
Carrier-free Gallium Citrate Ga 67 Injection has been found to concentrate in certain viable primary and metastatic tumors, as well as focal site of infection. The mechanism of concentration is unknown, but investigational studies have shown that Gallium Ga 67 accumulates in lysosomes and is bound to a soluble intracellular protein.
It has been reported in the scientific literature that following intravenous injection, the highest tissue concentration of Gallium Ga 67 – other than tumors and sites of infection– is in the renal cortex. After the first day, the maximum concentration shifts to bone and lymph nodes, and after the first week, to liver and spleen. Gallium is excreted relatively slowly from the body. The average whole body retention is 65% after 7 days, with 26% having been excreted in the urine and 9% in the stools.
-
INDICATIONS AND USAGES
Gallium Citrate Ga 67 Injection may be useful in demonstrating the presence of the following malignancies: Hodgkins disease, lymphomas and bronchogenic carcinoma. Positive Ga 67 uptake in the absence of prior symptoms warrants follow-up as an indication of a potential disease state.
Gallium Citrate Ga 67 Injection may be useful as an aid in detecting some acute inflammatory lesions.
- CONTRAINDICATIONS
-
WARNINGS
Because of the benzyl alcohol content, caution should be used in administration to newborns, particularly infants born prematurely, and individuals with impaired liver function.
The vial stopper contains dry natural rubber latex and may cause allergic reactions in providers or patients who are sensitive to latex.
-
PRECAUTIONS
General
A thorough knowledge of the normal distribution of intravenously administered Gallium Citrate Ga 67 Injection is essential in order to accurately interpret pathologic studies.
The finding in an abnormal gallium concentration usually implies the existence of underlying pathology, but further diagnostic studies should be done to distinguish benign from malignant lesions. Gallium Citrate Ga 67 Injection is intended for use as an adjunct in the diagnosis of certain neoplasms. Certain pathologic conditions may yield up to 40% false negative gallium studies. Therefore, a negative study cannot be definitively interpreted as ruling out the presence of disease.
Lymphocytic lymphoma frequently does not accumulate Gallium Ga 67 sufficiently for unequivocal imaging; and the use of gallium with this histologic type of lymphoma is not recommended at this time.
Gallium Ga 67 localization cannot differentiate between tumor and acute inflammation; and other diagnostic studies must be added to define the underlying pathology.
Gallium Citrate Ga 67 Injection, as well as any other radioactive drugs, must be handled with care, and appropriate safety measures should be used to minimize external radiation exposure to clinical personnel. Care should also be taken to minimize radiation exposure to patients consistent with proper patient management.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether Gallium Citrate Ga 67 Injection affects fertility in males or females.
Pregnancy
Animal reproductive studies have not been conducted with Gallium Citrate Ga 67 Injection. It is also not known whether Gallium Citrate Ga 67 Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Gallium Citrate Ga 67 Injection should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, in a woman of childbearing capability, should be performed during the first few (approximately 10) days following the onset of menses.
Nursing Mothers
Gallium Citrate Ga 67 Injection is excreted in human milk during lactation; therefore, formula feedings should be substituted for breast feedings.
Geriatric Use
Clinical studies of Gallium Citrate Ga67 Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The recommended adult (70 kg) dose of Gallium Citrate Ga 67 Injection is 74-185 MBq (2-5 mCi). Gallium Citrate Ga 67 Injection is intended for intravenous administration only.
Approximately 10% of the administered dose is excreted in the feces during the first week after injection. Daily laxatives and/or enemas are recommended during the first week after injection until the final images are obtained in order to cleanse the bowel of radioactive material and minimize the possibility of false positive studies.
Studies indicate the optimal tumor to background concentration ratios are often obtained about 48 hours post-injection. However, considerable biological variability may occur in individuals, and acceptable images may be obtained as early as 6 hours and as late as 120 hours after injection.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Waterproof gloves should be worn during the handling procedures. With a shielded sterile syringe, aseptically withdraw the material for use. The expiration date of the drug is fourteen days after the date of manufacture.
Radiation Dosimetry
The estimated absorbed radiation doses2 from an intravenous injection of 185 MBq (5 mCi) of Gallium Citrate Ga 67 are shown in Table 4.
TABLE 4. Dosimetry of Gallium Citrate Ga 67 Injection for Maximal Dose of 185 MBq (5 mCi) mGy/
185 MBqRads/
5mCiWhole Body 13.0 1.30 Skeleton 22.0 2.20 Liver 23.0 2.30 Bone Marrow 29.0 2.90 Spleen 26.5 2.65 Kidney 20.5 2.05 Ovaries 14.0 1.40 Testes 12.0 1.20 Gastrointestinal Tract Stomach 11.0 1.10 Small Intestine 18.0 1.80 Upper Large Intestine 28.0 2.80 Lower Large Intestine 45.0 4.50
- 2 MIRD Dose Estimate Report No.2, J. Nucl. Med. 14:755-6 (1973).
-
HOW SUPPLIED
Gallium Citrate Ga 67 Injection is supplied sterile and non-pyrogenic for intravenous use. Each mL contains 74 MBq (2 mCi) of Gallium Ga 67 on the calibration date, as a complex formed from 9 ng Gallium Chloride Ga 67, 2 mg of sodium citrate, 6.8 mg sodium chloride, and 9 mg benzyl alcohol/mL as preservative. The pH is adjusted to between 4.5-8 with hydrochloric acid and/or sodium hydroxide solution.
Vials are available in the following quantities of radioactivity: 244.2, 325.6, 488.4, and 732.6 MBq (6.6, 8.8, 13.2, and 19.8 mCi) of Gallium Citrate Ga 67 at calibration.
NDC Number 11994-121
Store at controlled room temperature 20°-25°C (68°-77°F) [See USP].
The contents of the vial are radioactive and adequate shielding and handling precautions must be maintained.
This radiopharmaceutical is approved for distribution to persons licensed pursuant to the Code of Massachusetts Regulations 105 CMR 120.100 for the uses listed in 105 CMR 120.547 or 120.552 or under equivalent regulations of the U.S. Nuclear Regulatory Commission, an Agreement State, or a Licensing State.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 2 mCi/mL Vial Label
515283-0811
Gallium
Gallium Citrate
Ga 67 InjectionSterile, Non-Pyrogenic Diagnostic Agent for Intravenous Injection
Contents & Assay: 74 MBq/mL
(2 mCi/mL), Gallium Chloride 9 ng/mL,
Sodium Citrate 2 mg/mL, Sodium
Chloride 6.8 mg/mL, Benzyl Alcohol
9 mg/mL. The pH is adjusted with
NaOH &/or HCl.
Rx only. Est. Lic. No.: 101647-A AUST R 19144
See Package Insert for dosing information.
Vial Stopper Contains Dry Natural Rubber LatexCAUTION: RADIOACTIVE MATERIAL
Manufacturer: Lantheus Medical Imaging, Inc. USA
Canadian License Holder: Lantheus MI Canada, Inc.
Australian Sponsor: Lantheus MI Australia Pty Ltd.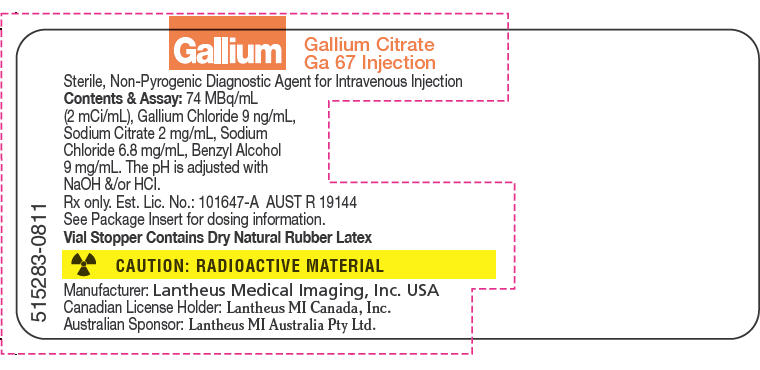
-
PRINCIPAL DISPLAY PANEL - 2 mCi/mL Vial Label
515284-0811
Gallium
Gallium Citrate
Ga 67 InjectionSterile, Non-Pyrogenic Diagnostic Agent for Intravenous Injection
Contents & Assay: 74 MBq/mL (2 mCi/mL), Gallium Chloride
9 ng/mL, Sodium Citrate 2 mg/mL, Sodium Chloride 6.8 mg/mL,
Benzyl Alcohol 9 mg/mL. The pH is adjusted with NaOH &/or HCl.Rx only. See Package Insert for dosing information.
Est. Lic. No.: 101647-A AUST R 19144Store at controlled room temperature 20°to 25°C (68°to 77°F) [see USP].
Vial Stopper Contains Dry Natural Rubber Latex
CAUTION: RADIOACTIVE MATERIAL
Manufacturer:
Lantheus Medical
Imaging, Inc.
N. Billerica, MA 01862 USACanadian Lic. Holder:
Lantheus MI Canada, Inc.
Montréal, CanadaAustralian Sponsor:
Lantheus MI Australia Pty Ltd.
Unit 8/24-26 Carrick Drive
Tullamarine VIC 3043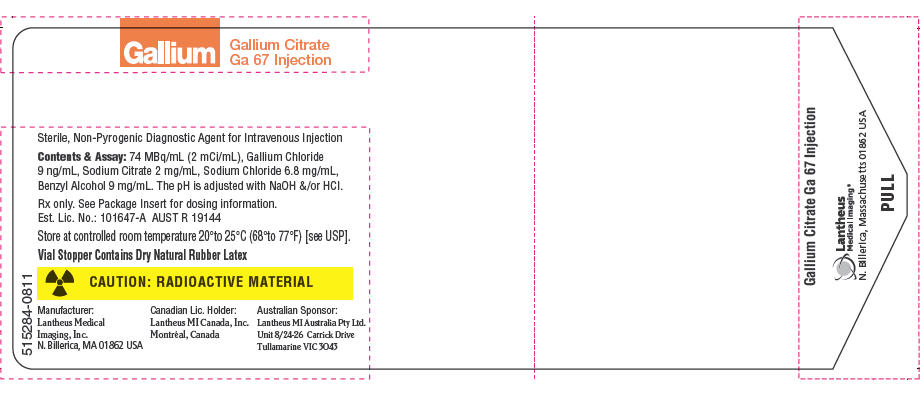
-
INGREDIENTS AND APPEARANCE
GALLIUM
gallium citrate ga-67 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11994-121 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GALLIUM CITRATE GA-67 (UNII: 4LJK511Z86) (GALLIUM CATION GA-67 - UNII:99T03J52W0) GALLIUM CATION GA-67 2.0 mCi in 1 mL Inactive Ingredients Ingredient Name Strength GALLIUM CHLORIDE GA-67 (UNII: A04B19O2B0) 9 ng in 1 mL SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZYL ALCOHOL (UNII: LKG8494WBH) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11994-121-06 3.3 mL in 1 VIAL; Type 0: Not a Combination Product 05/17/1976 2 NDC: 11994-121-08 4.4 mL in 1 VIAL; Type 0: Not a Combination Product 03/03/2004 3 NDC: 11994-121-13 6.6 mL in 1 VIAL; Type 0: Not a Combination Product 05/17/1976 4 NDC: 11994-121-19 9.9 mL in 1 VIAL; Type 0: Not a Combination Product 05/17/1976 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017478 05/17/1976 Labeler - Lantheus Medical Imaging, Inc. (176786812) Establishment Name Address ID/FEI Business Operations Lantheus Medical Imaging, Inc. 176786812 MANUFACTURE(11994-121) , LABEL(11994-121) , PACK(11994-121) , REPACK(11994-121) , RELABEL(11994-121)
Trademark Results [Gallium]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GALLIUM 98743262 not registered Live/Pending |
Zachary Rudman 2024-09-10 |
 GALLIUM 90602283 not registered Live/Pending |
Intelligent Automation, Inc. 2021-03-25 |
 GALLIUM 90009490 not registered Live/Pending |
Jihye Whang Rosenband 2020-06-18 |
 GALLIUM 86224585 4848911 Live/Registered |
CYCLES ARGON-18 INC. 2014-03-18 |
 GALLIUM 85303793 not registered Dead/Abandoned |
Caesars License Company, LLC 2011-04-25 |
 GALLIUM 78685378 not registered Dead/Abandoned |
Amazon Partners, LLC 2005-08-04 |
 GALLIUM 78029669 not registered Dead/Abandoned |
Runge Ll Christopher 2000-10-08 |
 GALLIUM 77033875 3281800 Dead/Cancelled |
Gallium Lighting 2006-11-01 |
 GALLIUM 74410633 2130817 Live/Registered |
KONGSBERG GEOSPATIAL LTD. 1993-07-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.