SENNA tablet, film coated
SENNA by
Drug Labeling and Warnings
SENNA by is a Otc medication manufactured, distributed, or labeled by HIMPRIT PHARMACHEM PVT LTD, SPIRIT PHARMACEUTICALS,LLC, ELYSIUM PHARMACEUTICALS LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
-
Directions
- take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children 12 years of age or older 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor - Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL - Shipping Label
Each Film Coated Tablet Contains :
CALCIUM SENNOSIDES 8.6mgLot No. :
Mfg. Date :
Exp. Date :
Jar No. :
Quantity : 50,000 Tablets
NDC No : 65437 - 087 - 50WARNING :
KEEP OUT OF THE REACH OF CHILDRENSTORE AT CONTROLLED ROOM TEMPERATURE OF 59°-77°F (15°-25° C )
PROTECT FROM LIGHT, MOISTURE AND FREEZING.THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE FDA AND REGULATIONS THEREUNDER.Manufactured By :
MANUFACTURER CODE No.: Guj/Drugs/G/1362
LABELER CODE # 14803Manufactured For.
HIMPRIT PHARMACHEM PVT. LTD.
"LAKULESH", R.V. DESAI ROAD,
NEXT TO NAVAPURA POLICE STATION,
BARODA, INDIA - 390 001CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"

-
INGREDIENTS AND APPEARANCE
SENNA
senna tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65437-087 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNA (UNII: AK7JF626KX) (SENNA - UNII:AK7JF626KX) SENNA 8.6 mg Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE (UNII: 3NXW29V3WO) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) Product Characteristics Color BROWN Score no score Shape ROUND Size 10mm Flavor Imprint Code S8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65437-087-50 1 in 1 DRUM 1 50000 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part334 11/20/2009 Labeler - HIMPRIT PHARMACHEM PVT LTD (917261992) Registrant - SPIRIT PHARMACEUTICALS,LLC (179621011) Establishment Name Address ID/FEI Business Operations ELYSIUM PHARMACEUTICALS LTD 858352983 MANUFACTURE
Trademark Results [SENNA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENNA 97868919 not registered Live/Pending |
Natty Collection LLC 2023-04-02 |
 SENNA 90583748 not registered Live/Pending |
Jeremey Decena 2021-03-17 |
 SENNA 90399285 not registered Live/Pending |
AYRTON SENNA EMPREENDIMENTOS LTDA. 2020-12-21 |
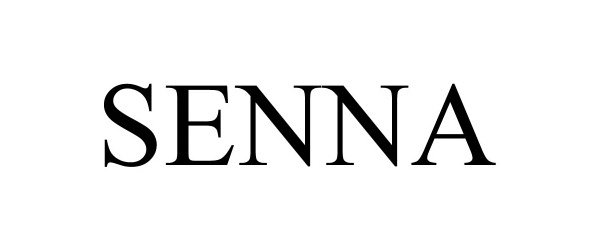 SENNA 90022160 not registered Live/Pending |
OPWEST DEVELOPMENT LLC 2020-06-26 |
 SENNA 88624114 not registered Live/Pending |
Ceritas Wines LLC 2019-09-19 |
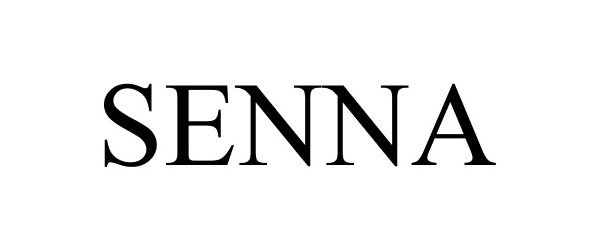 SENNA 87683504 5564030 Live/Registered |
OMM Imports Inc. 2017-11-14 |
 SENNA 76601884 3268781 Dead/Cancelled |
Studio RTA 2004-07-12 |
 SENNA 75170094 2188775 Live/Registered |
Senna Cosmetics, Inc. 1996-09-23 |
 SENNA 74561186 not registered Dead/Abandoned |
Senna Cosmetics, Inc. 1994-08-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.