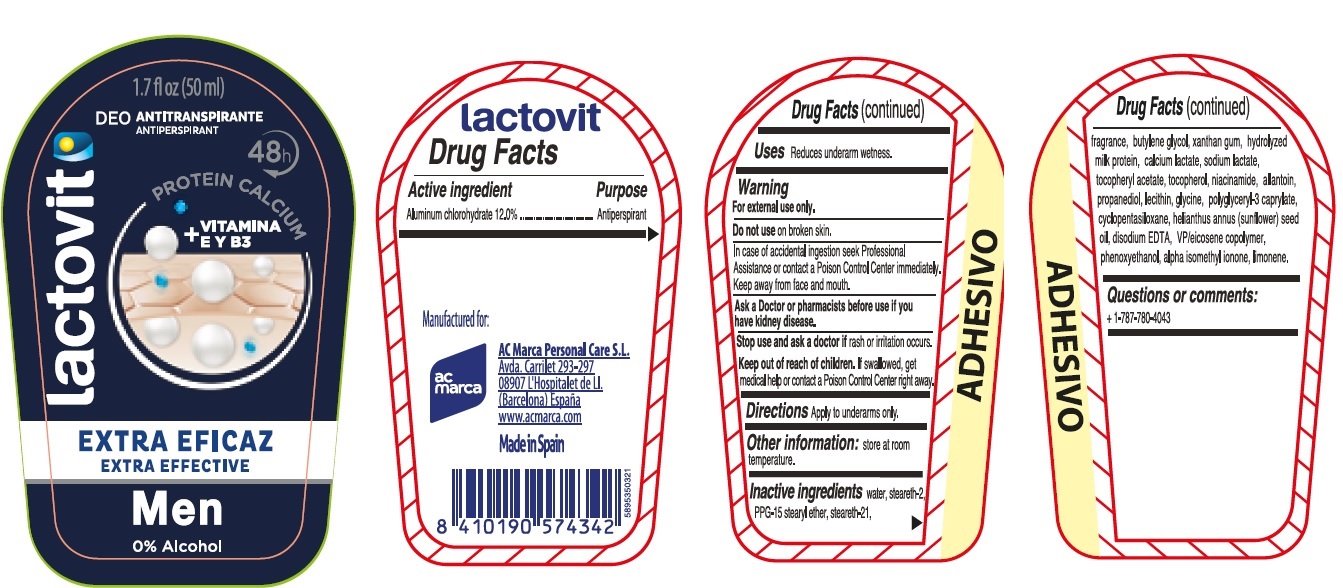
Active ingredient
Aluminum chlorohydrate 12.0%
Uses
Reduces underarm wetness.
Warning
For external use only.
Do not use
on broken skin.
In case of accidental ingestion seek Professional Assistance or contact a Poison Control Center immediately. Keep away from face and mouth.
Ask a Doctor or pharmacists
before use if you have kidney disease.
Stop use and ask a doctor if
rash or irritation occurs.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply to underarms only.
Other information:
Store at room temperature.
Inactive ingredients
water, Steareth-2, PPG-15 Stearyl Ether, Steareth-21, fragrance, butylene glycol, xanthan gum, hydrolyzed milk protein, Calcium lactate, Sodium lactate, tocopheryl acetate, tocopherol, niacinamide, allantoin, propanediol, lecithin,glycine, polyglyceryl-3caprylate,cyclopentasiloxane, helianthus annus (sunflower) seed oil, disodium EDTA, VP/eicosene copolymer, phenoxyethanol, alpha isomethyl iononel, linalool.
Questions or comments:
+ 1-787-780-4043
Package Labeling: