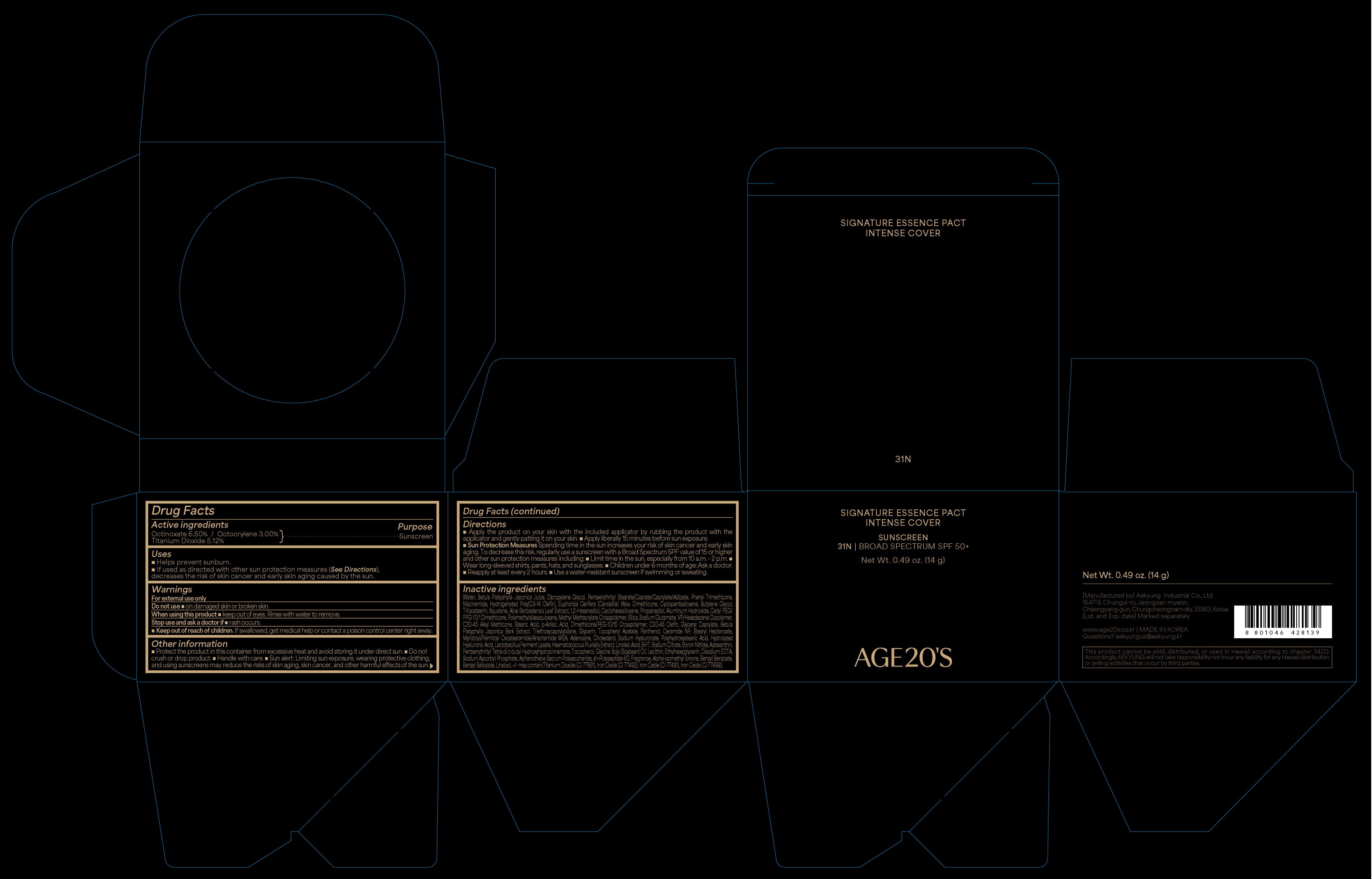
67225-5131-2 AGE 20s SIGNATURE ESSENCE PACT INTENSE COVER 31N
AGE 20s SIGNATURE ESSENCE COVER PACT INTENSE COVER 31 MEDIUM TAN by
Drug Labeling and Warnings
AGE 20s SIGNATURE ESSENCE COVER PACT INTENSE COVER 31 MEDIUM TAN by is a Otc medication manufactured, distributed, or labeled by Aekyung Industrial Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AGE 20S SIGNATURE ESSENCE PACT INTENSE COVER 31N- octinoxate, octocrylene, titanium dioxide cream
Aekyung Industrial Co., Ltd.
----------
67225-5131-2 AGE 20s SIGNATURE ESSENCE PACT INTENSE COVER 31N
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.
Directions
■ Apply the product on your skin with the included applicator by rubbing the product with the applicator and gently patting it on your skin.
■ Apply liberally 15 minutes before sun exposure.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
· Limit time in the sun, especially from 10 a.m. - 2 p.m.
· Wear long-sleeved shirts, pants, hats, and sunglasses.
■ Children under 6 months of age: Ask a doctor.
■ Reapply at least every 2 hours.
■ Use a water-resistant sunscreen if swimming or sweating.
Other information
■ Protect the product in this container from excessive heat and avoid storing it under direct sun.
■ Do not crush or drop product.
■ Handle with care.
■ Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
Betula Platyphylla Japonica Bark Extract, Betula Platyphylla Japonica Juice, Dipropylene Glycol, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Phenyl Trimethicone, Niacinamide, Hydrogenated Poly(C6-14 Olefin), Euphorbia Cerifera (Candelilla) Wax, Dimethicone, Cyclopentasiloxane, Butylene Glycol, Squalane, Aloe Barbadensis Leaf Extract, Triisostearin, 1,2-Hexanediol, Cyclohexasiloxane, Polymethylsilsesquioxane, Propanediol, Cetyl PEG/PPG-10/1 Dimethicone, Sodium Glutamate, VP/Hexadecene Copolymer, Aluminum Hydroxide, C30-45 Alkyl Methicone, p-Anisic Acid, Dimethicone/PEG-10/15 Crosspolymer, Stearic Acid, C30-45 Olefin, Glyceryl Caprylate, Glycerin, Triethoxycaprylylsilane, Alumina, Tocopheryl Acetate, Panthenol, Ceramide NP, Stearyl Heptanoate, Myristoyl/Palmitoyl Oxostearamide/Arachamide MEA, Adenosine, Cholesterol, Water, Sodium Hyaluronate, Polyhydroxystearic Acid, Hydrolyzed Hyaluronic Acid, Lactobacillus Ferment Lysate, Haematococcus Pluvialis Extract, Linoleic Acid, Boron Nitride, Astaxanthin, Aphanothece Sacrum Polysaccharide, Glycine Soja (Soybean) Oil, Lecithin, Ethylhexylglycerin, sh-Polypeptide-60, Fragrance, Alpha-Isomethyl Ionone, Benzyl Benzoate, Benzyl Salicylate, Linalool, +/- may contain (Titanium Dioxide (CI 77891), Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499))
| AGE 20S SIGNATURE ESSENCE PACT INTENSE COVER 31N
octinoxate, octocrylene, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Aekyung Industrial Co., Ltd. (690511126) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aekyung Industrial Co., Ltd. | 690511126 | manufacture(67225-5131) | |