DUREZOL- difluprednate emulsion
DUREZOL by
Drug Labeling and Warnings
DUREZOL by is a Prescription medication manufactured, distributed, or labeled by Sandoz Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DUREZOL safely and effectively. See full prescribing information for DUREZOL.
DUREZOL® (difluprednate ophthalmic emulsion) 0.05%, for topical ophthalmic use
Initial U.S. Approval: 2008INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For the treatment of inflammation and pain associated with ocular surgery, instill one drop into the conjunctival sac of the affected eye 4 times daily beginning 24 hours after surgery and continuing throughout the first 2 weeks of the postoperative period, followed by 2 times daily for a week and then a taper based on the response. (2.1)
- For the treatment of endogenous anterior uveitis instill one drop into the conjunctival sac of the affected eye 4 times daily for 14 days followed by tapering as clinically indicated. (2.2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic emulsion: 0.05%. (3)
CONTRAINDICATIONS
DUREZOL is contraindicated in most active viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. (4)
WARNINGS AND PRECAUTIONS
- Intraocular Pressure (IOP) Increase: Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. If DUREZOL is used for 10 days or longer, IOP should be monitored. (5.1)
- Cataracts: Use of corticosteroids may result in posterior subcapsular cataract formation. (5.2)
- Delayed Healing: The use of corticosteroids after cataract surgery may delay healing and increase the incidence of bleb formation. (5.3)
- Corneal and Scleral Melting: In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical corticosteroids. (5.4)
- Bacterial Infections: Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, corticosteroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be reevaluated. (5.5)
- Viral Infections: Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). (5.6)
- Fungal Infections: Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use. (5.7)
ADVERSE REACTIONS
For treatment of inflammation and pain associated with ocular surgery, most common adverse reactions (incidence 5% to 15%) are corneal edema, ciliary and conjunctival hyperemia, eye pain, photophobia, posterior capsule opacification, anterior chamber cells, anterior chamber flare, conjunctival edema, and blepharitis.
For treatment of endogenous anterior uveitis, most common adverse reactions (incidence 5% to 10%) are blurred vision, eye irritation, eye pain, headache, increased IOP, iritis, limbal and conjunctival hyperemia, punctate keratitis, and uveitis.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc., at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Ocular Surgery
1.2 Endogenous Anterior Uveitis
2 DOSAGE AND ADMINISTRATION
2.1 Ocular Surgery
2.2 Endogenous Anterior Uveitis
2.3 Prescribing Guidelines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Intraocular Pressure (IOP) Increase
5.2 Cataracts
5.3 Delayed Healing
5.4 Corneal and Scleral Melting
5.5 Bacterial Infections
5.6 Viral Infections
5.7 Fungal Infections
5.8 Topical Ophthalmic Use
5.9 Risk of Contamination
5.10 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Ocular Surgery
6.2 Endogenous Anterior Uveitis
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Ocular Surgery
14.2 Endogenous Anterior Uveitis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Ocular Surgery
Instill one drop into the conjunctival sac of the affected eye 4 times daily beginning 24 hours after surgery and continuing throughout the first 2 weeks of the postoperative period, followed by 2 times daily for a week and then a taper based on the response.
2.2 Endogenous Anterior Uveitis
Instill one drop into the conjunctival sac of the affected eye 4 times daily for 14 days followed by tapering as clinically indicated.
2.3 Prescribing Guidelines
The initial prescription and renewal of the medication order beyond one bottle should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and where appropriate, fluorescein staining. If signs and symptoms fail to improve after two days, the patient should be reevaluated.
Not more than one bottle should be prescribed initially, and the prescription should not be refilled without further evaluation.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DUREZOL, as with other ophthalmic corticosteroids, is contraindicated in most active viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal disease of ocular structures.
-
5 WARNINGS AND PRECAUTIONS
5.1 Intraocular Pressure (IOP) Increase
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Corticosteroids should be used with caution in the presence of glaucoma. If DUREZOL is used for 10 days or longer, IOP should be routinely monitored.
5.3 Delayed Healing
The use of corticosteroids after cataract surgery may delay healing and increase the incidence of bleb formation. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. The initial prescription and renewal of the medication order beyond 28 days should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and where appropriate, fluorescein staining.
5.4 Corneal and Scleral Melting
Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation of the globe.
5.5 Bacterial Infections
Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, corticosteroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be reevaluated.
5.6 Viral Infections
Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution; frequent slit lamp microscopy is recommended.
5.7 Fungal Infections
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal culture should be taken when appropriate.
5.9 Risk of Contamination
Do not allow the dropper tip to touch the eye, eyelids, or any surface, as this may contaminate the ophthalmic emulsion.
5.10 Contact Lens Wear
The anti-microbial preservative in DUREZOL may be absorbed by soft contact lenses. DUREZOL should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of DUREZOL. Contact lenses may be reinserted after 10 minutes following administration of DUREZOL.
-
6 ADVERSE REACTIONS
The following serious reactions are found elsewhere in the labeling:
- Intraocular Pressure (IOP) Increase [see Warnings and Precautions (5.1)]
- Cataracts [see Warnings and Precautions (5.2)]
- Delayed Healing [see Warnings and Precautions (5.3)]
- Corneal and Scleral Melting [see Warnings and Precautions (5.4)]
- Bacterial Infections [see Warnings and Precautions (5.5)]
- Viral Infections [see Warnings and Precautions (5.6)]
- Fungal Infections [see Warnings and Precautions (5.7)]
6.1 Ocular Surgery
Ocular adverse reactions occurring in 5% to 15% of subjects in clinical studies with DUREZOL, included corneal edema, ciliary and conjunctival hyperemia, eye pain, photophobia, posterior capsule opacification, anterior chamber cells, anterior chamber flare, conjunctival edema, and blepharitis. Other ocular adverse reactions occurring in 1% to 5% of subjects, included reduced visual acuity, punctate keratitis, eye inflammation, and iritis. Ocular adverse reactions occurring in less than 1% of subjects, included application-site discomfort or irritation, corneal pigmentation and striae, episcleritis, eye pruritus, eyelid irritation and crusting, foreign body sensation, increased lacrimation, macular edema, sclera hyperemia, and uveitis. Most of these reactions may have been the consequence of the surgical procedure.
6.2 Endogenous Anterior Uveitis
A total of 200 subjects participated in the clinical trials for endogenous anterior uveitis, of which 106 were exposed to DUREZOL. The most common adverse reactions of those exposed to DUREZOL occurring in 5% to 10% of subjects included blurred vision, eye irritation, eye pain, headache, increased IOP, iritis, limbal and conjunctival hyperemia, punctate keratitis, and uveitis. Adverse reactions occurring in 2% to 5% of subjects included anterior chamber flare, corneal edema, dry eye, iridocyclitis, photophobia, and reduced visual acuity.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on DUREZOL use in pregnant women to evaluate for a drug- associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
Systemic exposure to DUREZOL following ocular administration is low [see Clinical Pharmacology (12.3)]. Consequently, the systemic exposure of a pregnant woman to difluprednate is expected to be minimal following topical ocular administration.
In animal reproductive studies, subcutaneous administration of difluprednate to pregnant rabbits and rats throughout organogenesis produced maternal toxicity, embryo-fetal toxicity and teratogenicity in rabbits and fetotoxicity in rats.
Administration of difluprednate to rats during late gestation through lactation did not produce adverse maternal, fetal or neonatal effects at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In embryofetal development studies, subcutaneous administration of difluprednate to pregnant rats during the period of organogenesis decreased the placental weight, at doses greater than or equal to 10 mcg/kg/day (approximately 0.48-fold higher than the maximum recommended human ophthalmic dose [MRHOD] of 0.2 mg/day, on a mg/m2 basis and assuming 100% absorption of the dose). Decreased weight gain and delayed ossification of the fetus were observed at a dose of 100 mcg/kg/day (approximately 4.8-fold higher than the MRHOD). The no-observed-effect level (NOEL) for teratogenic effects was 10 mcg/kg/day. In rabbits, subcutaneous administration of difluprednate during the period of organogenesis produced maternal toxicity, embryofetal lethality, fetal growth retardation and teratogenicity (cleft palate, cerebral hernia, exencephaly, hypogenesis of the first digit of the forelimbs, club hand, umbilical hernia and others) at 10 mcg/kg/day (approximately 0.97-fold the MRHOD on a mg/m2 basis and assuming 100% absorption of the dose). The NOEL was 1 mcg/kg/day.
In a peri- or pre-/postnatal study in rats, subcutaneous administration of difluprednate during late gestation through lactation resulted in no abnormalities in postnatal development, growth and behavior or reproductive potential. The NOEL was greater than or equal to 10 mcg/kg/day (the highest dose tested; approximately 0.48-fold higher than the MRHOD on a mg/m2basis and assuming 100% absorption of the dose).
8.2 Lactation
Risk Summary
There are no data on the presence of DUREZOL in human milk, the effects on the breastfed infants, or the effects on milk production to inform risk of DUREZOL to an infant during lactation. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. The systemic exposure of a breastfeeding woman to difluprednate is expected to be minimal following topical ocular administration, however, the possibility of harm to the breastfed child cannot be ruled out.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DUREZOL and any potential adverse effects on the breast-fed child from DUREZOL or from the underlying maternal condition.
8.3 Nursing Mothers
It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when DUREZOL is administered to a nursing woman.
8.4 Pediatric Use
DUREZOL was evaluated in a 3-month multicenter, double-masked trial in 79 pediatric patients (39 DUREZOL; 40 prednisolone acetate) 0 to 3 years of age for the treatment of inflammation following cataract surgery. A similar safety profile was observed in pediatric patients comparing DUREZOL to prednisolone acetate ophthalmic suspension, 1%.
-
11 DESCRIPTION
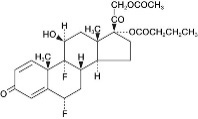
DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for topical ophthalmic use. The chemical name is 6α,9difluoro-11β,17,21-trihydroxypregna-1,4- diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-86-4). Difluprednate is represented by the following structural formula:
Difluprednate has a molecular weight of 508.56 g/mol, and the empirical formula is C27H34F2O7.
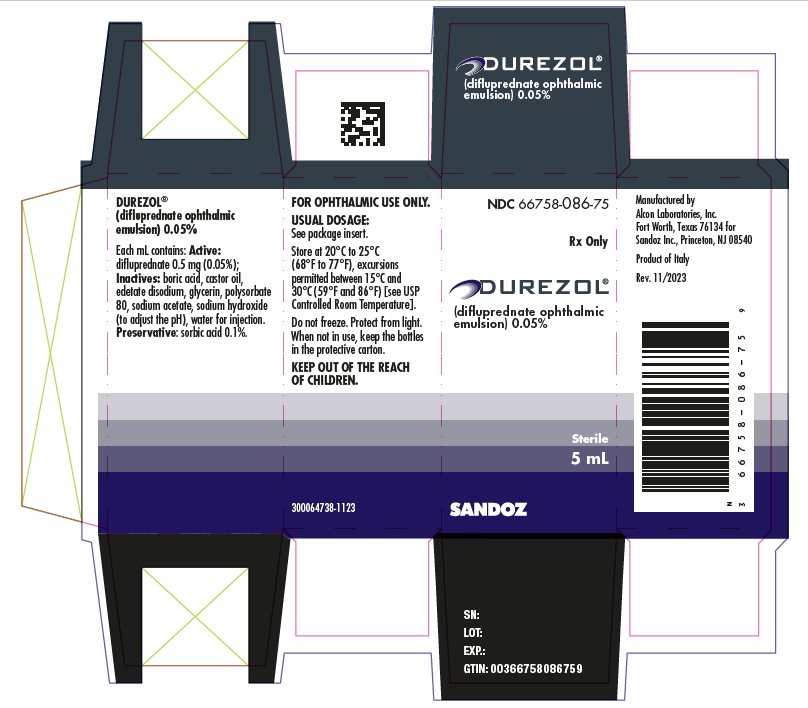
Each mL of DUREZOL contains: Active: difluprednate 0.5 mg (0.05%); Inactives: boric acid, castor oil, edetate disodium, glycerin, polysorbate 80, sodium acetate, sodium hydroxide (to adjust the pH to 5.2 to 5.8), water for injection. Preservative: sorbic acid 0.1%.
The emulsion is essentially isotonic with a tonicity of 304 to 411 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids inhibit the inflammatory response to a variety of inciting agents and may delay or slow healing. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation, such as prostaglandins and leukotreines by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Difluprednate is structurally similar to other corticosteroids.
12.3 Pharmacokinetics
Difluprednate undergoes deacetylation in vivo to 6α, 9-difluoroprednisolone 17-butyrate (DFB), an active metabolite of difluprednate.
Clinical pharmacokinetic studies of difluprednate after repeat ocular instillation of 2 drops of difluprednate (0.01% or 0.05%) 4 times per day for 7 days showed that DFB levels in blood were below the quantification limit (50 ng/mL) at all time points for all subjects, indicating that the systemic absorption of difluprednate after ocular instillation of DUREZOL is limited.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
Long-term studies have not been conducted to evaluate the carcinogenic potential of difluprednate.
Mutagenesis
Difluprednate was not genotoxic in vitro in the Ames test, and in cultured mammalian cells (CHL/IU; a fibroblastic cell line derived from the lungs of newborn female Chinese hamsters) or in an in vivo micronucleus test of difluprednate in mice.
Impairment of Fertility
Treatment of male and female rats with subcutaneous difluprednate up to 10 mcg/kg/day prior to and during mating did not impair fertility in either gender.
13.2 Animal Toxicology and/or Pharmacology
In multiple studies performed in rodents and non-rodents, subchronic and chronic toxicity tests of difluprednate showed systemic effects, such as suppression of body weight gain; a decrease in lymphocyte count; atrophy of the lymphatic glands and adrenal gland; and for local effects, thinning of the skin; all of which were due to the pharmacologic action of the molecule and are well known glucocorticosteroid effects. Most, if not all of these effects were reversible after drug withdrawal. The NOEL for the subchronic and chronic toxicity tests were consistent between species and ranged from 1-1.25 mcg/kg/day.
-
14 CLINICAL STUDIES
14.1 Ocular Surgery
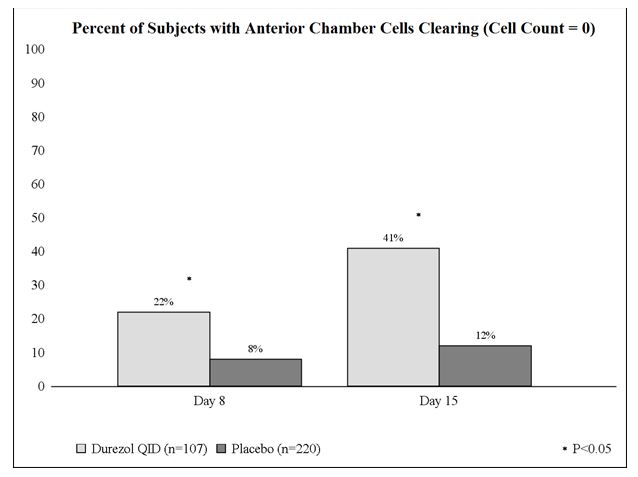
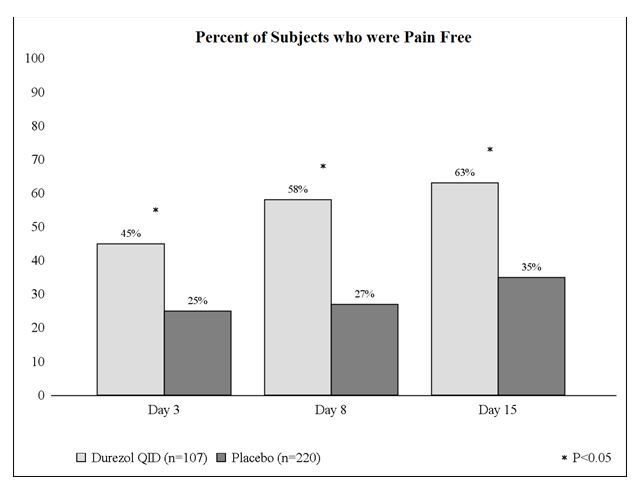
Clinical efficacy was evaluated in 2 randomized, double-masked, placebo-controlled trials in which subjects with an anterior chamber cell grade greater than or equal to "2" (a cell count of 11 or higher) after cataract surgery were assigned to DUREZOL or placebo (vehicle) following surgery. One drop of DUREZOL or vehicle was self-instilled either 2 times per day or 4 times per day for 14 days, beginning the day after surgery. The presence of complete clearing (a cell count of 0) was assessed 3, 8, and 15 days post surgery using a slit lamp binocular microscope. In the intent-to-treat analyses of both studies, a significant benefit was seen in the 4 times per day DUREZOL-treated group in ocular inflammation, at Days 8 and Day 15, and reduction of pain at Days 3, 8, and 15 when compared with placebo. The consolidated clinical trial results are provided below.
14.2 Endogenous Anterior Uveitis
Clinical efficacy was evaluated in two randomized, double masked active controlled trials in which patients who presented with endogenous anterior uveitis were treated with either DUREZOL 4 times daily or prednisolone acetate ophthalmic suspension 1%, 8 times daily for 14 days. Both studies demonstrated that DUREZOL was equally effective as prednisolone acetate ophthalmic suspension 1% in treating subjects with endogenous anterior uveitis. The results are found in Table 1 below.
Table 1: Mean Change From Baseline in Anterior Chamber Cell Grade* Abbreviation: CI, confidence interval.
*With 5 grades: 0 = 0 cells; 1 = 1 to 10 cells; 2 = 11 to 20 cells; 3 = 21 to 50 cells; and 4 = greater than 50 cells.
†Adjusted for baseline AC cell grade and study center and based on ITT dataset with LOCF for missing data.Study 1
Time PointDUREZOL
N = 57Prednisolone Acetate
N = 53Difference†
(95% CI)Baseline
2.6
2.5
0.0 (-0.22, 0.28)
Day 3
-1.0
-1.0
-0.1 (-0.35, 0.25)
Day 7
-1.6
-1.5
0.0 (-0.31, 0.25)
Day 14
-2.0
-1.8
-0.2 (-0.46, 0.10)
Day 21
-2.2
-1.9
-0.3 (-0.53, 0.01)
Day 28
-2.2
-2.1
-0.1 (-0.37, 0.18)
Day 35
-2.1
-2.0
-0.1 (-0.39, 0.20)
Day 42
-2.1
-2.1
0.0 (-0.27, 0.34)
Study 2
Time PointDUREZOL
N = 50Prednisolone Acetate
N = 40Difference†
(95% CI)Baseline
2.4
2.4
0.0 (-0.21, 0.29)
Day 3
-0.9
-0.9
0.0 (-0.34, 0.25)
Day 7
-1.7
-1.6
-0.1 (-0.35, 0.21)
Day 14
-1.9
-1.8
-0.1 (-0.34, 0.20)
Day 21
-2.0
-2.0
0.0 (-0.25, 0.28)
Day 28
-2.0
-2.0
0.0 (-0.21, 0.26)
Day 35
-2.1
-2.0
-0.1 (-0.32, 0.16)
Day 42
-2.0
-1.9
-0.1 (-0.36, 0.24)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, aqueous topical ophthalmic emulsion supplied in an opaque plastic bottle with a controlled drop tip and a pink cap in the following sizes:
5 mL in a 8 mL bottle NDC: 66758-086-75
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Do not freeze. Protect from light. When not in use, keep the bottles in the protective carton. After opening, DUREZOL can be used until the expiration date on the bottle.
-
17 PATIENT COUNSELING INFORMATION
When to Consult a Physician
Advise patients to consult a physician if pain develops, or if redness, itching, or inflammation becomes aggravated [see Warnings and Precautions (5.5)].
Risk of Contamination
Advise patients to not allow the dropper tip to touch the eye, eyelids, or any surface, as this may contaminate the ophthalmic emulsion.
Contact Lens Wear
Advise patients to not use DUREZOL while wearing contact lenses. Advise patients to remove contact lenses prior to instillation of DUREZOL. Lenses may be reinserted after 10 minutes following administration of DUREZOL [see Warnings and Precautions (5.9)].
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc., Princeton, NJ 08540
xxxxxx
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DUREZOL
difluprednate emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66758-086 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIFLUPREDNATE (UNII: S8A06QG2QE) (DIFLUPREDNATE - UNII:S8A06QG2QE) DIFLUPREDNATE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) CASTOR OIL (UNII: D5340Y2I9G) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE DISODIUM (UNII: 7FLD91C86K) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66758-086-75 1 in 1 CARTON 12/06/2024 1 5 mL in 1 BOTTLE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022212 01/25/2011 Labeler - Sandoz Inc (005387188)
Trademark Results [DUREZOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DUREZOL 77830018 3784208 Live/Registered |
NOVARTIS AG 2009-09-18 |
 DUREZOL 77340065 not registered Dead/Abandoned |
Sirion Therapeutics, Inc. 2007-11-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.