GENTLE LAXATIVE- bisacodyl tablet, sugar coated
Gentle Laxative by
Drug Labeling and Warnings
Gentle Laxative by is a Otc medication manufactured, distributed, or labeled by Wal-Mart Stores Inc, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
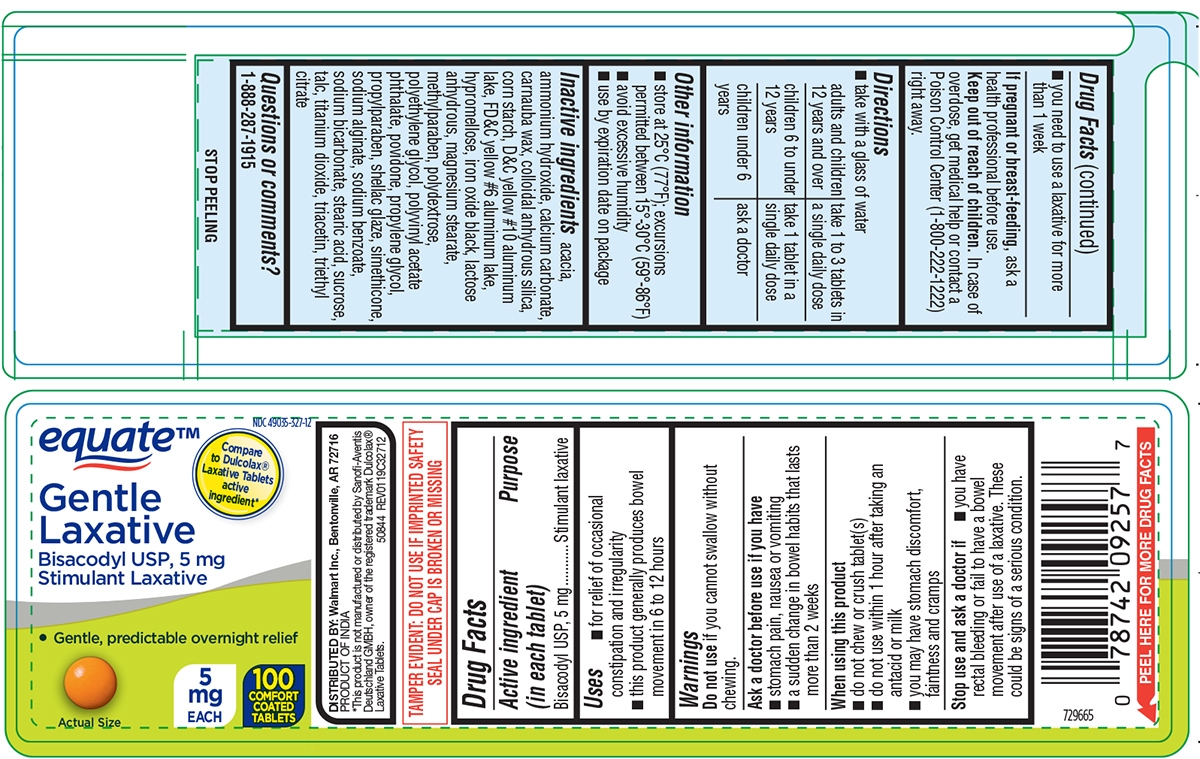
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- you may have stomach discomfort, faintness and cramps
- Directions
- Other information
-
Inactive ingredients
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, lactose anhydrous, magnesium stearate, methylparaben, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparaben, shellac glaze, simethicone, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
-
Principal Display Panel
NDC: 49035-327-12
equate™
Compare to Dulcolax® Laxative Tablets active ingredient*
Gentle
Laxative
Bisacodyl USP, 5 mg
Stimulant LaxativeGentle, predictable overnight relief
5
mg
EACH100
COMFORT
COATED
TABLETSActual size
DISTRIBUTED BY: Walmart Inc., Bentonville, AR 72716
PRODUCT OF INDIA
* This product is not manufactured or distributed by Sanofi-Aventis Deutschland GMBH, owner of the registered trademark Dulcolax® Laxative Tablets.
50844 REV0119C32712TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Equate 44-327
-
INGREDIENTS AND APPEARANCE
GENTLE LAXATIVE
bisacodyl tablet, sugar coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49035-327 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 5 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) AMMONIA (UNII: 5138Q19F1X) CALCIUM CARBONATE (UNII: H0G9379FGK) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM ALGINATE (UNII: C269C4G2ZQ) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Polyvinyl Acetate Phthalate (UNII: 58QVG85GW3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) Product Characteristics Color ORANGE Score no score Shape ROUND Size 6mm Flavor Imprint Code 5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49035-327-19 1 in 1 CARTON 03/25/2002 05/24/2016 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 49035-327-12 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/25/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 03/25/2002 Labeler - Wal-Mart Stores Inc (051957769) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(49035-327) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(49035-327) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(49035-327) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(49035-327) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(49035-327)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.