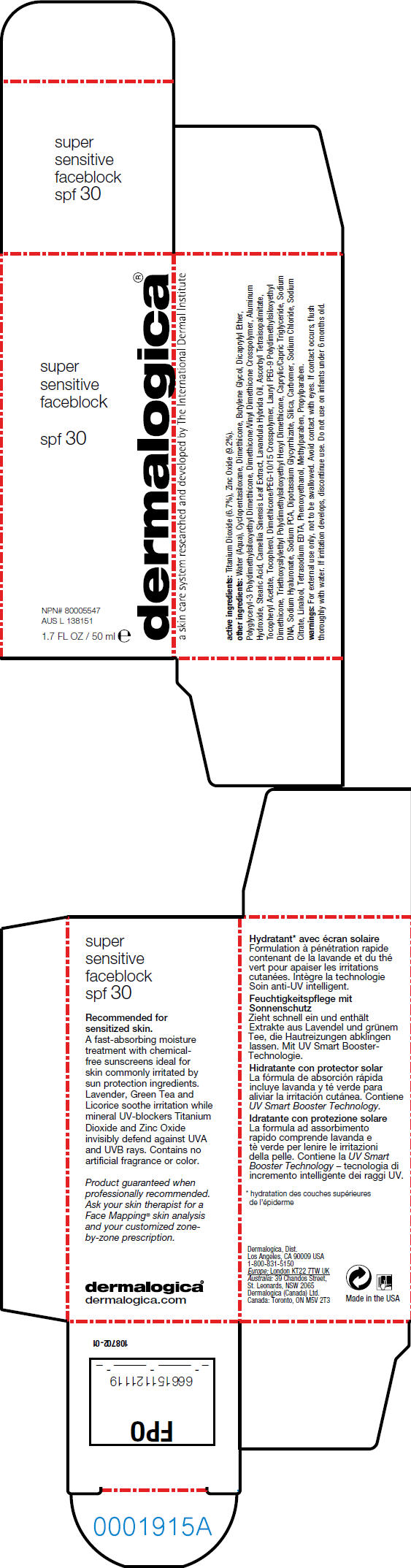
FACEBLOCK- titanium dioxide and zinc oxide lotion
FACEBLOCK by
Drug Labeling and Warnings
FACEBLOCK by is a Otc medication manufactured, distributed, or labeled by Gordon Laboratories, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- active ingredients
-
other ingredients
Water (Aqua), Cyclopentasiloxane, Dimethicone, Butylene Glycol, Dicaprylyl Ether, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Aluminum Hydroxide, Stearic Acid, Camellia Sinensis Leaf Extract, Lavandula Hybrida Oil, Ascorbyl Tetraisopalmitate, Tocopheryl Acetate, Tocopherol, Dimethicone/PEG-10/15 Crosspolymer, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Caprylic/Capric Triglyceride, Sodium DNA, Sodium Hyaluronate, Sodium PCA, Dipotassium Glycyrrhizate, Silica, Carbomer, Sodium Chloride, Sodium Citrate, Linalool, Tetrasodium EDTA, Phenoxyethanol, Methylparaben, Propylparaben.
- warnings
- PRINCIPAL DISPLAY PANEL - 50 ml Carton
-
INGREDIENTS AND APPEARANCE
FACEBLOCK SPF30 SUPER SENSITIVE
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 21839-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.35 mL in 50 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.6 mL in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Dimethicone (UNII: 92RU3N3Y1O) Butylene Glycol (UNII: 3XUS85K0RA) Dicaprylyl Ether (UNII: 77JZM5516Z) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Stearic Acid (UNII: 4ELV7Z65AP) Green Tea Leaf (UNII: W2ZU1RY8B0) Lavandin Oil (UNII: 9RES347CKG) Alpha-Tocopherol (UNII: H4N855PNZ1) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Hyaluronate Sodium (UNII: YSE9PPT4TH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sodium Chloride (UNII: 451W47IQ8X) Sodium Citrate (UNII: 1Q73Q2JULR) Linalool, Dl- (UNII: D81QY6I88E) Edetate Sodium (UNII: MP1J8420LU) Phenoxyethanol (UNII: HIE492ZZ3T) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21839-111-50 1 in 1 BOX 1 50 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 11/09/2005 Labeler - Gordon Laboratories, Inc (008328619)
Trademark Results [FACEBLOCK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FACEBLOCK 87617572 5479702 Live/Registered |
Faceblock, LLC 2017-09-21 |
 FACEBLOCK 78524114 not registered Dead/Abandoned |
mary kay roney 2004-11-29 |
 FACEBLOCK 78037471 not registered Dead/Abandoned |
Newtech Commercialization Ltd. 2000-12-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.