NEUT- sodium bicarbonate injection, solution
Neut by
Drug Labeling and Warnings
Neut by is a Prescription medication manufactured, distributed, or labeled by Hospira, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Neut (4% sodium bicarbonate additive solution) is a sterile, nonpyrogenic solution of sodium bicarbonate in water for injection. It is administered by the intravenous route only after addition as a neutralizing agent to an acidic large volume parenteral solution. Each 5 mL contains sodium bicarbonate 0.2 g (2.4 mEq each of Na+ and HCO3‾ ); edetate disodium, anhydrous 10 mg added as a stabilizer. Total sodium (Na+) content of each 5 mL is 56.1 mg (11.2 mg/mL).
The solutions contain no bacteriostat, antimicrobial agent or added buffer; pH 8.0 (7.0 to 8.5).
Sodium Bicarbonate, USP is chemically designated as NaHCO3, a white crystalline powder soluble in water.
-
CLINICAL PHARMACOLOGY
The acid pH of most intravenous solutions has been implicated as a factor in the production of postinfusion (chemical) phlebitis not caused by obvious infection. Vein irritation, with local redness and tenderness near the site of venipuncture or along the course of a vein, appears to be related to the nature of the substances in the infusion and the speed (insufficient dilution by the bloodstream) as well as the duration (prolonged exposure of the intima) of infusion. Other contributing factors include the size of the vein used for venipuncture, shape or method of insertion of the venipuncture needle, the use or type of indwelling catheter, infection at the infusion site and the age of the patient (children and females seem to be more susceptible).
The pH of commonly used dextrose infusion solutions ranges from 3.5 to 6.5. Other commonly used solutions also may have an acid pH. Since non-neutral parenteral solutions with a low (acid) pH are known to cause chemical irritation of tissues, it is not surprising that chemical phlebitis may occur as a complication with their infusion. Vein irritation is most likely when the duration of infusion is long or when hemodilution is minimized by a large needle in a small vein.
The amount of sodium bicarbonate recommended as an additive to neutralize acid parenteral solutions is too small to exert a clinically significant increase in electrolyte content.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Solutions prepared with Neut (4% sodium bicarbonate additive solution) should be administered promptly. When introducing additives, use aseptic technique, mix thoroughly and do not store.
When Neut is added to Hospira solutions, the compatibility of these solutions with other drugs may be altered. (See section on COMPATIBILITY, etc., for listing of additives tested with Neut added D5-W.)
Raising the pH of I.V. fluids with Neut will only reduce the incidence of chemical irritation caused by the infusate; it will not diminish any foreign body effects caused by the needle or catheter.
Pregnancy
Animal reproduction studies have not been conducted with sodium bicarbonate. It is also not known whether sodium bicarbonate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium bicarbonate should be given to a pregnant woman only if clearly needed.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
One vial (5 mL) of Neut added to a liter (1000 mL) of any of the following Hospira parenteral solutions will increase the pH to a more physiologic range. Specific pH may vary slightly from lot to lot.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
Product List 5% Alcohol in 5% Dextrose Injection, USP 1500 2.5% Dextrose Inj., USP 1508 5% Dextrose Inj., USP 1522 10% Dextrose Inj., USP 1530 20% Dextrose Inj., USP 1535 Ringer's Injection, USP 1582 0.9% Sodium Chloride Inj., USP 1583 Sodium Lactate Inj., USP (1/6 Molar) 1587 Addition of one vial of Neut to one-half liter (500 mL) is recommended to achieve a more physiologic pH of the following Hospira parenteral solutions:
Product List 6% Dextran 70 and 0.9% Sodium Chloride Injection 1505 6% Dextran 70 and 5% Dextrose Injection 1507 Note: Some products, e.g., Aminosyn™ solutions and those Ionosol™ and Normosol™ formulas containing dextrose will NOT be brought to near physiologic pH by the addition of Neut. This is due to the relatively high buffer capacity of these fluids.
COMPATIBILITY & EFFECTIVENESS OF NEUT WITH ADDITIVES TO 5% DEXTROSE INJECTION (D5-W)
When medications are added to intravenous solutions, the resultant admixtures may or may not be compatible in solutions containing Neut (4% sodium bicarbonate additive solution).
Following is a list of medications each added to one liter of 5% Dextrose Injection, USP (D5-W) classified according to their effect with Neut (4% sodium bicarbonate additive solution).
1. Neut Compatible with Admixtures for 24 Hours Conc./liter - * Requires 2 vials of Neut to bring admixture to approximate neutrality.
ACTHAR® (ACTH) 40 units Aqua-Mephyton® (Vitamin K1) 10 mg Aramine® (metaraminol) Bitartrate 100 mg* Atropine Sulfate 0.4 mg Calcium Chloride 1 g Calcium Gluceptate 1.1 g Compazine® (prochlorperazine) Edisylate 10 mg Crystodigin® (digitoxin) 0.2 mg Demerol® (meperidine) HCl 100 mg Ergonovine Maleate 0.2 mg Erythrocin® (erythromycin) Lactobionate 1 g Fungizone® (amphotericin B) 50 mg Ilotycin® (erythromycin) Gluceptate 1 g Innovar® (droperidol and fentanyl) 1.0 mL Keflin® (cephalothin) Sodium 4 g Lidocaine HCl 1 g M.V.I.™ (multivitamin infusion) 10 mL* Neosynephrine® (phenylephrine) HCl 10 mg Panheparin® (heparin sodium) 20,000 units Penicillin G Potassium 100,000,000 units Pitocin® (oxytocin) 5 units Potassium Chloride 120 mEq Sodium Iodide 1 g (promazine) HCl 100 mg Vancocin® (vancomycin) HCl 500 mg Wydase® (hyaluronidase) 150 units 2. Neut Incompatible –Additive Inactivated at Neutral pH Conc./liter Epinephrine 4 mg Isuprel™ (isoproterenol) HCl 1 mg Levophed™ (levarterenol) Bitartrate 8 mg Quelicin™ (succinylcholine chloride) 1 g 3. Neut Ineffective -Additive High in Titratable Acidity Conc./liter Achromycin® (tetracycline) HCl 500 mg Aureomycin® (chlortetracycline) HCl 500 mg Bejectal w/C (B complex and C) 10 mL Kantrex® (kanamycin) Sulfate 1 g 4. Neut Not Indicated –Admixture Already At or Near Neutrality Conc./liter Gantrisin® (sulfisoxazole) Diethanolamine 4 g Hydrocortone® (hydrocortisone) Phosphate 100 mg Prostaphlin® (oxacillin) Sodium 500 mg Sodium Bicarbonate 3.75 g Solu-Cortef ® (hydrocortisone sodium succinate) 250 mg 5. Neut Not Indicated –Admixture Already Alkaline Conc./liter Aminophylline 500 mg Amytal® (amobarbital) Sodium 500 mg Dilantin® (diphenylhydantoin) Sodium 250 mg Diuril® (chlorothiazide) 0.5 g Emivan® (ethimivan) 2 g Furadantin® (nitrofurantoin) Sodium 180 mg Nembutal® (pentobarbital) Sodium 500 mg Pentothal™ (thiopental sodium) 1 g Phenobarbital Sodium 320 mg Polycillin® N (ampicillin sodium) 500 mg Sulfadiazine Sodium 1 g It should be noted that the admixtures were evaluated for physical compatibility, not for pharmacological compatibility. It, therefore, would be erroneous to circumvent medical judgment which must be involved in administering any solution that appears to be compatible on the basis of having no visible haze or precipitate. The inclusion of drugs in this study of their compatibility in solution does not imply their therapeutic usefulness or safety. This matter remains the judgment of the prescribing physician.
NOTE: The compatibility information contained herein is based on the studies involving Hospira dextrose only. Variations in compatibility could occur due to lot-to-lot variations or formula changes in the additives or dextrose solutions of other manufacturers.
-
HOW SUPPLIED
Neut (sodium bicarbonate 4% additive solution), single-dose glass fliptop vial
NDC: 0409-6609-25: 2.4 mEq/5 mL (0.48 mEq/mL), supplied in a 5 mL partial-fill single-dose, glass fliptop vial.
- SPL UNCLASSIFIED SECTION
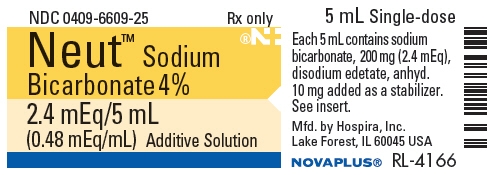
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial Tray
25 Units/NDC: 0409-6609-25
5 mL Single-doseNeut™
Sodium Bicarbonate 4%Rx only
2.4 mEq/5 mL (0.48 mEq/mL)
Additive SolutionCA–6150

-
INGREDIENTS AND APPEARANCE
NEUT
sodium bicarbonate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-6609 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37, BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 0.2 g in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-6609-25 25 in 1 TRAY 03/05/2014 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 03/05/2014 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-6609) , LABEL(0409-6609) , MANUFACTURE(0409-6609) , PACK(0409-6609)
Trademark Results [Neut]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEUT 97793986 not registered Live/Pending |
Innogized Technologies, Inc. 2023-02-14 |
 NEUT 78045255 not registered Dead/Abandoned |
ASPEngines, Inc. 2001-01-26 |
 NEUT 72325939 0888942 Live/Registered |
ABBOTT LABORATORIES 1969-04-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.