CORAXIS TOPICAL SOLUTION- moxidectin solution
Coraxis by
Drug Labeling and Warnings
Coraxis by is a Animal medication manufactured, distributed, or labeled by Bayer HealthCare, LLC Animal Health Division. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
CORAXISTM
(moxidectin)
Topical Solution for Dogs
Once-a-month topical solution for the prevention of heartworm disease caused by Dirofilaria immitis, as well as the treatment and control of intestinal hookworm, roundworm and whipworm infections in dogs and puppies that are at least 7 weeks of age and that weigh at least 3 lbs.
-
BOXED WARNING
(What is this?)
WARNING
- DO NOT ADMINISTER THIS PRODUCT ORALLY
- For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
- Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information)
- CAUTION:
-
DESCRIPTION:
CORAXIS™ (2.5% moxidectin) is a colorless to yellow, ready-to-use solution packaged in single dose applicator tubes for topical treatment of dogs. The formulation and dosage schedule are designed to provide a minimum of 1.1 mg/lb (2.5 mg/kg) moxidectin based on body weight.
Moxidectin is a semisynthetic macrocyclic lactone endectocide derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus. The chemical name for moxidectin is [6R,23E,25S(E)]-5-O-Demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-(methoxyimino) milbemycin B.
-
INDICATIONS:
CORAXIS is indicated for the prevention of heartworm disease caused by Dirofilaria immitis. CORAXIS is also indicated for the treatment and control of the following intestinal parasites:
Intestinal Parasite
Intestinal Stage
Adult
Immature
Adult
Fourth Stage
Larvae
Hookworm
Species
Ancylostoma
caninum
X
X
X
Uncinaria
stenocephala
X
X
X
Roundworm
Species
Toxocara canis
X
X
Toxascaris
leonina
X
Whipworm
Trichuris vulpis
X
-
CONTRAINDICATIONS:
Do not administer this product orally. (See WARNINGS.)
Do not use this product (containing 2.5% moxidectin) on cats.
-
WARNINGS:
For the first 30 minutes after application:
Ensure that dogs cannot lick the product from application sites on themselves or other treated dogs, and separate treated dogs from one another and from other pets to reduce the risk of accidental ingestion.
Ingestion of this product by dogs may cause serious adverse reactions including depression, salivation, dilated pupils, incoordination, panting, and generalized muscle tremors.
In avermectin sensitive dogs,a the signs may be more severe and may include coma and death.b
a Some dogs are more sensitive to avermectins due to a mutation in the ABCB1 gene (formerly MDR1 gene). Dogs with this mutation may develop signs of severe avermectin toxicity if they ingest this product. The most common breeds associated with this mutation include Collies and Collie crosses.
b Although there is no specific antagonist for avermectin toxicity, even severely affected dogs have completely recovered from avermectin toxicity with intensive veterinary supportive care.
-
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children.
Children should not come in contact with application sites for two (2) hours after application. Causes eye irritation. Harmful if swallowed. Do not get in eyes or on clothing. Avoid contact with skin. Exposure to the product has been reported to cause headache; dizziness; and redness, burning, tingling, or numbness of the skin. Wash hands thoroughly with soap and warm water after handling.
If contact with eyes occurs, hold eyelids open and flush with copious amounts of water for 15 minutes. If eye irritation develops or persists, contact a physician. If swallowed, call poison control center or physician immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or physician. People with known hypersensitivity to benzyl alcohol or moxidectin should administer the product with caution. In case of allergic reaction, contact a physician. If contact with skin or clothing occurs, take off contaminated clothing. Wash skin immediately with plenty of soap and water. Call a poison control center or physician for treatment advice.
The Safety Data Sheet (SDS) provides additional occupational safety information. For a copy of the Safety Data Sheet (SDS) or to report adverse reactions call Bayer Veterinary Services at 1-800-422-9874. For consumer questions call 1-800-255-6826.
-
PRECAUTIONS:
Do not dispense dose applicator tubes without complete safety and administration information.
Use with caution in sick, debilitated, or underweight animals. The safety of CORAXIS has not been established in breeding, pregnant, or lactating dogs. The safe use of CORAXIS has not been established in puppies and dogs less than 7 weeks of age or less than 3 lbs body weight.
Prior to administration of CORAXIS, dogs should be tested for existing heartworm infection. At the discretion of the veterinarian, infected dogs should be treated with an adulticide to remove adult heartworms.
CORAXIS is not effective against adult D. immitis. (See ANIMAL SAFETY – Safety Study in Heartworm-Positive Dogs.) -
ADVERSE REACTIONS:
Since CORAXIS contains 2.5% moxidectin, studies that demonstrated the safe use of a topical solution containing 2.5% moxidectin + 10% imidacloprid were acceptable to demonstrate the safety of CORAXIS.
Field Studies: Following treatment with a topical solution containing 2.5% moxidectin + 10% imidacloprid or an active control, dog owners reported the following post-treatment reactions:
OBSERVATION
Moxidectin + Imidacloprid
n = 128
Active Control
n = 68
Pruritus
19 dogs (14.8%)
7 dogs (10.3%)
Residue
9 dogs (7.0%)
5 dogs (7.4%)
Medicinal Odor
5 dogs (3.9%)
None observed
Lethargy
1 dog (0.8%)
1 dog (1.5%)
Inappetence
1 dog (0.8%)
1 dog (1.5%)
Hyperactivity
1 dog (0.8%)
None observed
During a field study of a topical solution containing 2.5% moxidectin + 10% imidacloprid using 61 dogs with pre-existing flea allergy dermatitis, one (1.6%) dog experienced localized pruritus immediately after product application, and one investigator noted hyperkeratosis at the application site of one dog (1.6%).
Laboratory Effectiveness Studies: One dog in a laboratory effectiveness study experienced weakness, depression and unsteadiness between 6 and 9 days after application of a topical solution containing 2.5% moxidectin + 10% imidacloprid. The signs resolved without intervention by day 10 post- application. The signs in this dog may have been related to peak serum levels of moxidectin, which vary between dogs, and occur between 1 and 21 days after product application.
The following clinical observations also occurred in laboratory effectiveness studies following application of a topical solution containing 2.5% moxidectin + 10% imidacloprid and may be directly attributed to the drug or may be secondary to the intestinal parasite burden or other underlying conditions in the dogs: diarrhea, bloody stools, vomiting, anorexia, lethargy, coughing, ocular discharge and nasal discharge. Observations at the application sites included damp, stiff or greasy hair, the appearance of a white deposit on the hair, and mild erythema, which resolved without treatment within 2 to 48 hours.
Foreign Market Experience: Because the following events were reported voluntarily during post-approval use of the product containing 2.5% moxidectin + 10% imidacloprid in foreign markets, it is not always possible to reliably establish a causal relationship to drug exposure. Adverse events associated with CORAXIS (2.5% moxidectin) are expected to be similar.
The following adverse events were reported in humans: eye irritation, allergic reactions, skin irritation, skin tingling, sore throat and chemical odor. Adverse events reported in dogs topically treated with a topical solution containing 2.5% moxidectin + 10% imidacloprid included: vomiting, diarrhea, bloody diarrhea, salivation, poor appetite, lethargy, weakness, restlessness, agitation, disorientation, ataxia, muscle tremors, seizures, panting, labored breathing, acute pulmonary edema, hives, rash, swollen face and ears, pruritus, erythema, alopecia, hot spots, local discomfort and discoloration of the hair at the application site. Accidental oral ingestion in dogs caused salivation, vomiting, muscle tremor, seizures, mydriasis, ataxia, lethargy, disorientation, agitation and poor appetite.
Adverse events in cats topically treated with imidacloprid + moxidectin for dogs included application site and skin reactions, vomiting, lethargy, agitation and neurologic signs.
To report a suspected adverse reaction, call 1-800-422-9874.
-
DOSAGE AND ADMINISTRATION:
The recommended minimum dose is 1.1 mg/lb (2.5 mg/kg) moxidectin, once-a-month, by topical administration.
Do not apply to irritated skin.
- 1. Remove one dose applicator tube from the package. As specified in the following table, administer the entire contents of the CORAXIS tube that correctly corresponds with the body weight of the dog.
* Dogs over 110 lbs. should be treated with the appropriate combination of CORAXIS tubes. Dog Weight
(lbs)
CORAXIS
Volume
(mL)
Moxidectin
(mg)
3–9
CORAXIS 9
0.4
10
9.1–20
CORAXIS 20
1.0
25
20.1–55
CORAXIS 55
2.5
62.5
55.1–88
CORAXIS 88
4.0
100
88.1–110*
CORAXIS 110
5.0
125
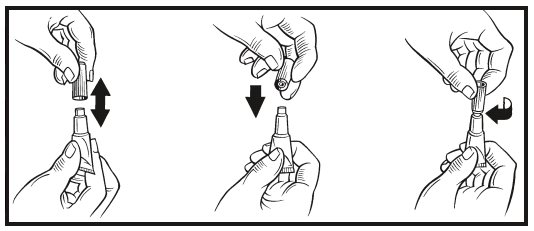
- 2. While holding the tube in an upright position, remove the cap from the tube.
- 3. Turn the cap over and push the other end of cap onto the tip of the tube.
- 4. Twist the cap to break the seal and then remove cap from the tube.
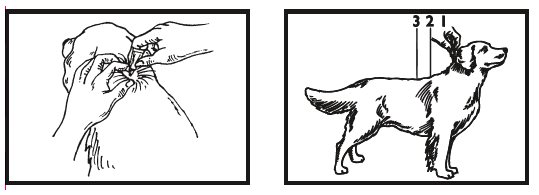
- 5. The dog should be standing for application. Part the hair on the back of the dog between the shoulder blades until the skin is visible. For dogs weighing 20 lbs or less, place the tip of the tube on the skin and apply the entire contents directly on the exposed skin at one spot between the shoulder blades. For dogs weighing more than 20 lbs, place the tip of the tube on the skin and apply the entire contents directly on the exposed skin at 3 or 4 spots on the top of the backline from the base of the neck to the upper back in an area inaccessible to licking. Do not apply an amount of solution at any one location that could run off the side of the dog.
Do not let this product get in your dog’s mouth or eyes. Do not allow the dog to lick any of the application sites for 30 minutes. In households with multiple pets, keep each treated dog separated from other treated dogs and other pets for 30 minutes after application to prevent licking the application sites. (See WARNINGS.)
Stiff hair, a damp appearance of the hair, pink skin or a slight powdery residue may be observed at the application site on some animals. This is temporary and does not affect the safety and effectiveness of the product.
Shampooing 90 minutes after treatment does not reduce the effectiveness of CORAXIS in the prevention of heartworm disease.
Heartworm Prevention: For prevention of heartworm disease, CORAXIS should be administered at one-month intervals. CORAXIS may be administered year-round or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer CORAXIS immediately and resume the monthly dosing schedule. When replacing another heartworm preventative product in a heartworm prevention program, the first treatment with CORAXIS should be given within one month of the last dose of the former medication.
Treatment and Control of Intestinal Nematode Infections: For the treatment and control of intestinal hookworm infections caused by Ancylostoma caninum and Uncinaria stenocephala (adults, immature adults and fourth-stage larvae) and roundworm infections caused by Toxocara canis (adults and fourth-stage larvae), and Toxascaris leonina (adults), and whipworm infections caused by Trichuris vulpis (adults), CORAXIS should be administered once as a single topical dose.
-
ANIMAL SAFETY:
In a controlled, double-masked, field safety study, a topical solution containing 2.5% moxidectin + 10% imidacloprid was administered to 128 dogs of various breeds, 3 months to 15 years of age, weighing 4 to 157 pounds. The moxidectin + imidacloprid topical solution was used safely in dogs concomitantly receiving ACE inhibitors, anticonvulsants, antihistamines, antimicrobials, chondroprotectants, corticosteroids, immunotherapeutics, MAO inhibitors, NSAIDs, ophthalmic medications, sympathomimetics, synthetic estrogens, thyroid hormones, and urinary acidifiers. Owners reported the following signs in their dogs after application of moxidectin + imidacloprid topical solution: pruritus, flaky/greasy residue at the treatment site, medicinal odor, lethargy, inappetence and hyperactivity. (See ADVERSE REACTIONS.)
Safety Study in Puppies: A solution containing 2.5% moxidectin + 10% imidacloprid was applied topically at 1, 3 and 5x the recommended dose to 7-week-old Beagle puppies once every 2 weeks for 6 treatments on days 0, 14, 28, 42, 56, and 70. Loose stools and diarrhea were observed in all groups, including the controls, throughout the study. Vomiting was seen in one puppy from the 1x treatment group (day 57), in two puppies from the 3x treatment group (days 1 and 79), and in one puppy from the 5x treatment group (day 1). Two puppies each in the 1x, 3x and 5x groups had decreased appetites within 24 hours post-dosing. One puppy in the 1x treatment group had pruritus for one hour following the fifth treatment. A puppy from the 5x treatment group displayed rapid, difficult breathing from 4 to 8 hours following the second treatment.
Dermal Dose Tolerance Study: A solution containing 2.5% moxidectin + 10% imidacloprid was administered topically to 8-month-old Beagle dogs once at 10x the recommended dose. One dog showed signs of treatment site irritation after application. Two dogs vomited, one at 6 hours and one at 6 days post-treatment. Increased RBC, hemoglobin, activated partial thromboplastin and direct bilirubin were observed in the treated group. Dogs in the treated group did not gain as much weight as the control group.
Safety Study in Heartworm-Positive Dogs: A solution containing 2.5% moxidectin + 10% imidacloprid was administered topically at 1 and 5x the recommended dose every 14 days for 3 treatments to dogs with adult heartworm infections and circulating microfilariae. At 5x, one dog was observed vomiting three hours after the second treatment. Hypersensitivity reactions were not seen in the 5x treatment group. Microfilariae counts decreased with treatment.
Oral Safety Study in Beagles: A solution containing 2.5% moxidectin + 10% imidacloprid was administered once orally at the recommended topical dose to 12 dogs. Six dogs vomited within 1 hour of receiving the test article, 2 of these dogs vomited again at 2 hours, and 1 dog vomited again at 18 hours post-dosing. One dog exhibited shaking (nervousness) 1 hour post-dosing. Another dog exhibited abnormal neurological signs (circling, ataxia, generalized muscle tremors and dilated pupils with a slow pupillary light response) starting at 4 hours post-dosing through 18 hours post-dosing. Without treatment, this dog was neurologically normal at 24 hours and had a normal appetite by 48 hours post-dosing. (See CONTRAINDICATIONS.)
Dermal Safety Study in Ivermectin-Sensitive Collies: A solution containing 2.5% moxidectin + 10% imidacloprid was administered topically at 3 and 5x the recommended dose every 28 days for 3 treatments to Collies which had been pre-screened to confirm avermectin sensitivity. No clinical abnormalities were observed.
Oral Safety Study in Ivermectin-Sensitive Collies: A solution containing 2.5% moxidectin + 10% imidacloprid was administered orally to 5 pre-screened ivermectin-sensitive Collies. The Collies were asymptomatic after ingesting 10% of the minimum labeled dose. At 40% of the minimum recommended topical dose, 4 of the dogs experienced neurological signs indicative of avermectin toxicity including depression, ataxia, mydriasis, salivation, muscle fasciculation and coma, and were euthanized. (See CONTRAINDICATIONS.)
- STORAGE INFORMATION:
-
HOW SUPPLIED:
Applications Per Package
6 x 0.4 mL tubes
6 x 1.0 mL tubes
6 x 2.5 mL tubes
6 x 4.0 mL tubes
6 x 5.0 mL tubesNADA # 141-417, Approved by FDA
Made in Italy
©2018 Bayer HealthCare LLC
Bayer (reg’d), the Bayer Cross (reg’d) and Coraxis™ are trademarks of Bayer.86572354
LV1808Bayer HealthCare LLC
Animal Health Division
P.O. Box 390, Shawnee Mission, Kansas 66201 U.S.A. -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 88.1-110 lbs dogs
6 Pack
88.1 - 110 lbsCORAXISTM
(moxidectin)Topical Solution for Dogs
Once-a-month topical solution for the prevention of heartworm disease caused by Dirofilaria immitis, as well as the treatment and control of hookworm, roundworm and whipworm infections in dogs and puppies that are at least 7 weeks of age and weigh 88.1 to 110 lbs.
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING
- DO NOT ADMINISTER THIS PRODUCT ORALLY
- For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
- Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information)
Each tube contains 125 mg of moxidectin in a 2.5% moxidectin solution.
Keep this and all drugs out of reach of children.
6 PACK
SIX 5.0 mL TUBESNADA # 141-417
Approved by FDA -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 55.1-88 lbs dogs
6 Pack
55.1 - 88 lbsCORAXISTM
(moxidectin)Topical Solution for Dogs
Once-a-month topical solution for the prevention of heartworm disease caused by Dirofilaria immitis, as well as the treatment and control of hookworm, roundworm and whipworm infections in dogs and puppies that are at least 7 weeks of age and weigh 55.1 to 88 lbs.
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING
- DO NOT ADMINISTER THIS PRODUCT ORALLY
- For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
- Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information)
Each tube contains 100 mg of moxidectin in a 2.5% moxidectin solution.
Keep this and all drugs out of reach of children.
6 PACK
SIX 4.0 mL TUBESNADA # 141-417
Approved by FDA -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 20.1-55 lbs dogs
6 Pack
20.1 - 55 lbsCORAXISTM
(moxidectin)Topical Solution for Dogs
Once-a-month topical solution for the prevention of heartworm disease caused by Dirofilaria immitis, as well as the treatment and control of hookworm, roundworm and whipworm infections in dogs and puppies that are at least 7 weeks of age and weigh 20.1 to 55 lbs.
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING
- DO NOT ADMINISTER THIS PRODUCT ORALLY
- For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
- Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information)
Each tube contains 62.5 mg of moxidectin in a 2.5% moxidectin solution.
Keep this and all drugs out of reach of children.
6 PACK
SIX 2.5 mL TUBESNADA # 141-417
Approved by FDA -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 9.1-20 lbs dogs
6 Pack
9.1 - 20 lbsCORAXISTM
(moxidectin)Topical Solution for Dogs
Once-a-month topical solution for the prevention of heartworm disease caused by Dirofilaria immitis, as well as the treatment and control of hookworm, roundworm and whipworm infections in dogs and puppies that are at least 7 weeks of age and weigh 9.1 to 20 lbs.
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING
- DO NOT ADMINISTER THIS PRODUCT ORALLY
- For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
- Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information)
Each tube contains 25 mg of moxidectin in a 2.5% moxidectin solution.
Keep this and all drugs out of reach of children.
6 PACK
SIX 1.0 mL TUBESNADA # 141-417
Approved by FDA -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 3-9 lbs dogs
6 Pack
3 - 9 lbsCORAXISTM
(moxidectin)Topical Solution for Dogs
Once-a-month topical solution for the prevention of heartworm disease caused by Dirofilaria immitis, as well as the treatment and control of hookworm, roundworm and whipworm infections in dogs and puppies that are at least 7 weeks of age and weigh 3 to 9 lbs.
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
WARNING
- DO NOT ADMINISTER THIS PRODUCT ORALLY
- For the first 30 minutes after application ensure that dogs cannot lick the product from application sites on themselves or other treated animals.
- Children should not come in contact with application sites for two (2) hours after application.
(See Contraindications, Warnings, Human Warnings, and Adverse Reactions, for more information)
Each tube contains 10 mg of moxidectin in a 2.5% moxidectin solution.
Keep this and all drugs out of reach of children.
6 PACK
SIX 0.4 mL TUBESNADA # 141-417
Approved by FDA -
INGREDIENTS AND APPEARANCE
CORAXIS TOPICAL SOLUTION
moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2416 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2416-02 2 in 1 CARTON 1 3 in 1 BLISTER PACK 1 NDC: 0859-2416-01 5 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141417 01/14/2019 CORAXIS TOPICAL SOLUTION
moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2417 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2417-02 1 in 1 CARTON 1 6 in 1 BLISTER PACK 1 NDC: 0859-2417-01 4 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141417 01/14/2019 CORAXIS TOPICAL SOLUTION
moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2418 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2418-02 1 in 1 CARTON 1 6 in 1 BLISTER PACK 1 NDC: 0859-2418-01 2.5 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141417 01/14/2019 CORAXIS TOPICAL SOLUTION
moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2419 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2419-02 1 in 1 CARTON 1 6 in 1 BLISTER PACK 1 NDC: 0859-2419-01 1 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141417 01/14/2019 CORAXIS TOPICAL SOLUTION
moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2420-02 1 in 1 CARTON 1 6 in 1 BLISTER PACK 1 NDC: 0859-2420-01 0.4 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141417 01/14/2019 Labeler - Bayer HealthCare, LLC Animal Health Division (152266193)
Trademark Results [Coraxis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CORAXIS 88071456 5759420 Live/Registered |
Bayer HealthCare LLC 2018-08-09 |
 CORAXIS 76318821 not registered Dead/Abandoned |
Sanjeev Batta 2001-10-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.