INDIUM 111 OXYQUINOLONE solution
Indium 111 Oxyquinolone by
Drug Labeling and Warnings
Indium 111 Oxyquinolone by is a Prescription medication manufactured, distributed, or labeled by AnazaoHealth Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
This preparation is supplied as a sterile, non-pyrogenic, isotonic aqueous solution with a pH range of 6.5 to 7.5 and is not for direct injection. Each milliliter contains 2 mCi at calibration time, 50μg oxyquinoline, 100μg polysorbate 80, and 6 mg of HEPES (N-2-hydroxyethyl-piperazine-N’-2-ethane sulfonic acid) buffer in 0.75% sodium chloride solution. It is intended for single use only and contains no bacteriostatic agent.
-
CLINICAL PHARMACOLOGY
Indium forms a saturated (1:3) complex with oxyquinoline. The complex is neutral and lipid-soluble, which enables it to penetrate the cell membrane. Within the cell, indium becomes firmly attached to cytoplasmic components; the liberated oxyquinoline is released by the cell. It is thought that the mechanism of labeling cells with Indium In-111 Oxyquinoline involves an exchange reaction between the oxyquinoline carrier and subcellular components, which chelate indium more strongly than oxyquinoline. The low stability constant of the oxyquinoline complex, estimated at approximately 10, supports this theory.
Following the recommended leukocyte cell labeling procedure, approximately 77% of the added Indium In-111Oxyquinoline is incorporated in the resulting cell pellet (which represents approximately 3-4 x 108 WBC).
Cell clumping can occur and was found in about one fifth of the leukocyte preparations examined. The presence of red blood cells or plasma will lead to reduced leukocyte labeling efficiency. Transferrin in plasma competes for Indium In-111 Oxyquinoline.
Chemotaxis of granulocytes deteriorates during storage and loss of chemotaxis may cause false negative scans. The spontaneous release of Indium In-111 has been reported to range from about 3% at one hour to 24% at 24 hours [ten Berge, R.J.M., Natarajan, A.T., Hardeman, M.R., et al. Labeling with Indium In-111 has detrimental effects on human lymphocytes. Journal of Nuclear Medicine, 24, 615-620 (1983)]. The maximum amount of time recommended between drawing the blood and reinjection should not exceed 5 hours. It is recommended that the labeled cells be used within one hour of preparation, if possible, and no more than three hours after preparation.
Cell aggregates of various degrees have been reported. Cell labeling techniques and standing of cell preparation may be contributing factors.
- DECAY CHARACTERISTICS
-
GENERAL
Strict aseptic techniques should be used to maintain sterility throughout the procedures for using this preparation.
Do not use after the expiration time and date (5 days after calibration) stated on the label. The contents of the vial are radioactive. Adequate shielding of the preparation must be maintained at all times.
Indium In-111 Oxyquinoline, like other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should also be taken to minimize radiation exposure to the patient consistent with proper patient management. Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides
-
DOSAGE AND ADMINISTRATION
The recommended adult (70kg) dose of Indium In-111 Oxyquinoline labeled autologous leukocytes is 7.4 to 18.5 MBq (200-500 μCi). Indium In-111 Oxyquinoline solution is intended for radiolabeling of autologous leukocytes. The Indium In-111 Oxyquinoline labeled autologous leukocytes are administered intravenously.
Imaging is recommended at approximately 24 hours post injection. Typically, anterior and posterior views of the chest, abdomen and pelvis should be obtained with other views as required. Aseptic procedures and a shielded syringe should be employed in the withdrawal of Indium In-111 Oxyquinoline from the vial. Similar procedures should be employed during the labeling procedure and the administration of the labeled leukocytes to the patient. The user should wear waterproof gloves during the entire procedure. The patient’s dose should be measured by a suitable radioactivity calibration system immediately before administration. At this time, the leukocyte preparation should be checked for gross clumping and red blood cell contamination
-
LABELING PROCEDURES
Sterile technique must be used throughout. It is important that all equipment used for the preparation of reagents be thoroughly cleaned to assure the absence of trace metal impurities. The user should wear waterproof gloves during the handling and administration procedure
- Withdraw from the patient 30-50 ml blood [preferably fifty (50) ml] using aseptic venipuncture technique using the 60 ml syringe fitted with a 19-gauge or 20-gauge needle and containing approximately 1000-1500 units heparin in 1-2 ml. Blood withdrawal should be smooth and slow so as not to produce bubbles or foaming
- Remove and dispose of the needle and replace with syringe cap. Gently mix the contents of the syringe and label with the patient’s ID, date and time.
- Upon receipt of the full syringe for processing, the contents should again be gently mixed.
- Clamp the syringe barrel to the ring stand in an upright (needle side up) position and tilt the syringe 10-20 degrees from its position perpendicular to the bench.
- Allow the red cells to sediment 30-60 minutes, depending upon when the supernatant [Leukocyte Rich Plasma (LRP)] looks clear of red cells.
- Replace the syringe cap with an infusion set
- Collect the plasma (LRP) in the centrifuge tube marked “WBC” by expressing the LRP through the catheter tubing making sure not to get any red cells into the WBC tube
- Immediately centrifuge the capped WBC tube at 400-450 g for 5 minutes
- Transfer the supernatant to the leukocyte poor plasma “LPP” tube leaving behind 0.5-1.0 ml supernatant to cover the white cell button. (Note: the button often contains a small number of red cells and may appear red.)
- Wash the white cell button with 4-6 ml Sodium Chloride (0.9%) Injection USP. Resuspend the cells by gentle swirling.
- Centrifuge the capped “WBC” tube at 400-450 g for 5 minutes (alternatively, 150 g for 8 minutes) and discard all but 0.5-1.0 ml of the supernatant to cover the cells.
- Add 5.0 ml Sodium Chloride (0.9%) Injection USP. Resuspend cells by gentle swirling.
- With the shielded syringe, draw up approximately 22.2 MBq (600 μCi) Indium In-111 Oxyquinoline. Check the amount of radioactivity in a dose calibrator set for Indium In-111 and record for labeling efficiency calculations. Parenteral drug preparations should be inspected visually for particulate matter and discoloration before administration.
- In several additions, add the Indium In-111 Oxyquinoline to the “WBC” tube, gently swirling after each addition.
- Set the lab timer for 15 minutes and allow the capped “WBC” tube to incubate. Swirl the cell preparation several times during the incubation.
- With a sterile plastic syringe, add half of the saved LPP (or about 8 ml) from the LPP tube. Cap and gently swirl the contents of “WBC” tube to resuspend the cells
- Centrifuge the “WBC” tube at 450 g for 5 minutes (or 150 g for 8 minutes). Decant supernatant into the “Wash” tube leaving behind about 0.5 ml of the supernate to cover the cells.
- Assay the activity in the “WBC” tube and in the “Wash” tube in a dose calibrator and record.
- With a sterile plastic syringe add the remaining LPP to the cell button and gently resuspend by swirling. With a sterile syringe fitted with a 19-gauge needle, resuspend the cells by drawing the cells up into the syringe and expressing the suspension against the tube gently one or twice. Alternatively, draw up the cells into a syringe fitted with the filtered 19-gauge needle, and replace the needle with an unfiltered 19- or 20-gauge needle.
- . Reserve in the “WBC” tube a minimum amount of white cell suspension for a WBC count. A microscopic examination should also be completed to observe for clumping. Draw up the patient’s dose 7.4 to 18.5 MBq (200-500 μCi) and check the syringe in the dose calibrator. Record the measurement
- QUALITY CONTROL
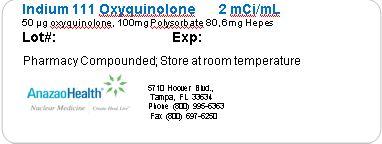
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INDIUM 111 OXYQUINOLONE
indium 111 oxyquinolone solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51808-126 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDIUM IN-111 OXYQUINOLINE (UNII: LGX9OL562T) (INDIUM IN-111 OXYQUINOLINE - UNII:LGX9OL562T) INDIUM IN-111 OXYQUINOLINE 2 mCi in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 100 ug in 1 mL HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID (UNII: RWW266YE9I) 6 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51808-126-01 1 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/19/2012 Labeler - AnazaoHealth Corporation (011038762) Establishment Name Address ID/FEI Business Operations AnazaoHealth Corporation 011038762 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.