BranTussin DM by Brandywine Pharmaceuticals, LLC BranTussin DM
BranTussin DM by
Drug Labeling and Warnings
BranTussin DM by is a Otc medication manufactured, distributed, or labeled by Brandywine Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
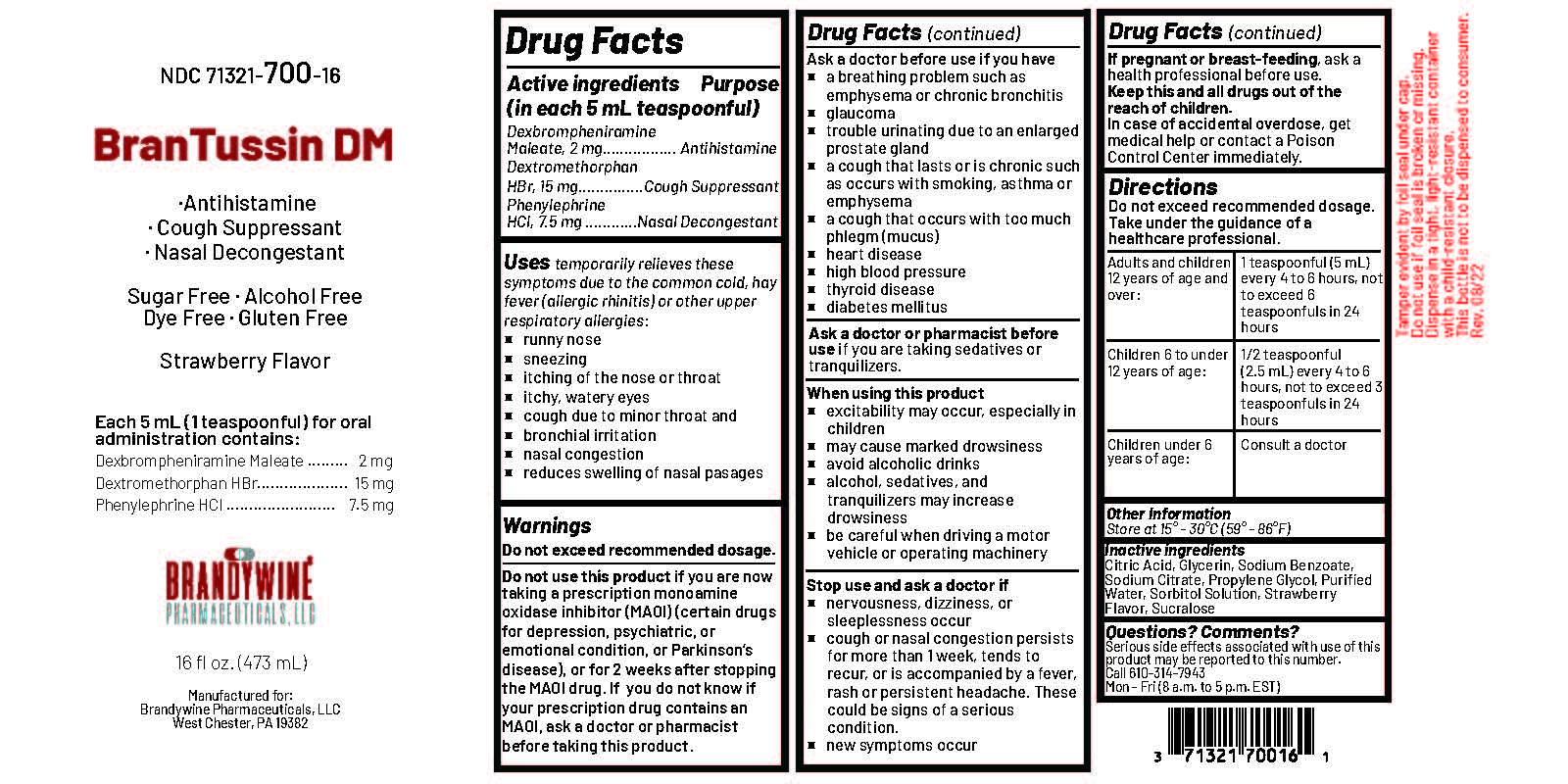
BRANTUSSIN DM- dexbrompheniramine maleate, dextromethorphan hbr, phenylephrine hcl liquid
Brandywine Pharmaceuticals, LLC
----------
BranTussin DM
Active Ingredient
Dexbrompheniramine Maleate 2 mg
Dextromethorphan Hydrobromide 15 mg
Phenylephrine Hydrochloride 7.5 mg
Uses
temporarily relieves these symptoms due to the common cold, hayfever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- cough due to minor throat and
- bronchial irritation
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use this product
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional condition, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
Stop use and ask a doctor if
nervousness, dizziness, or sleeplessness occur
cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. A persistent cough may be a sign of a serious condition.
new symptoms occur
Keep this and all drugs out of the reach of children
In case of accidental overdose, get medical help or contact a Poison Control Center immediately.
Directions
Do not exceed recommended dosage
| Adults and children 12 years of age and over | 1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 6 teaspoonfuls in 24 |
| Children 6 to under 12 years of age | 1/2 teaspoonful (2.5 mL) every 4 to 6 hours, not to exceed 3 teaspoonfuls in 24 hours |
| Children under 6 years of age | Consult a doctor. |
Inactive Ingredients
Anhydrous Citric Acid, Glycerin, Flavor, Propylene Glycol, Purified Water, Sodium Benzoate, Sodium Citrate Dihydrate, Sodium Saccharin, Sorbitol Solution.
| BRANTUSSIN DM
dexbrompheniramine maleate, dextromethorphan hbr, phenylephrine hcl liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Brandywine Pharmaceuticals, LLC (080581956) |