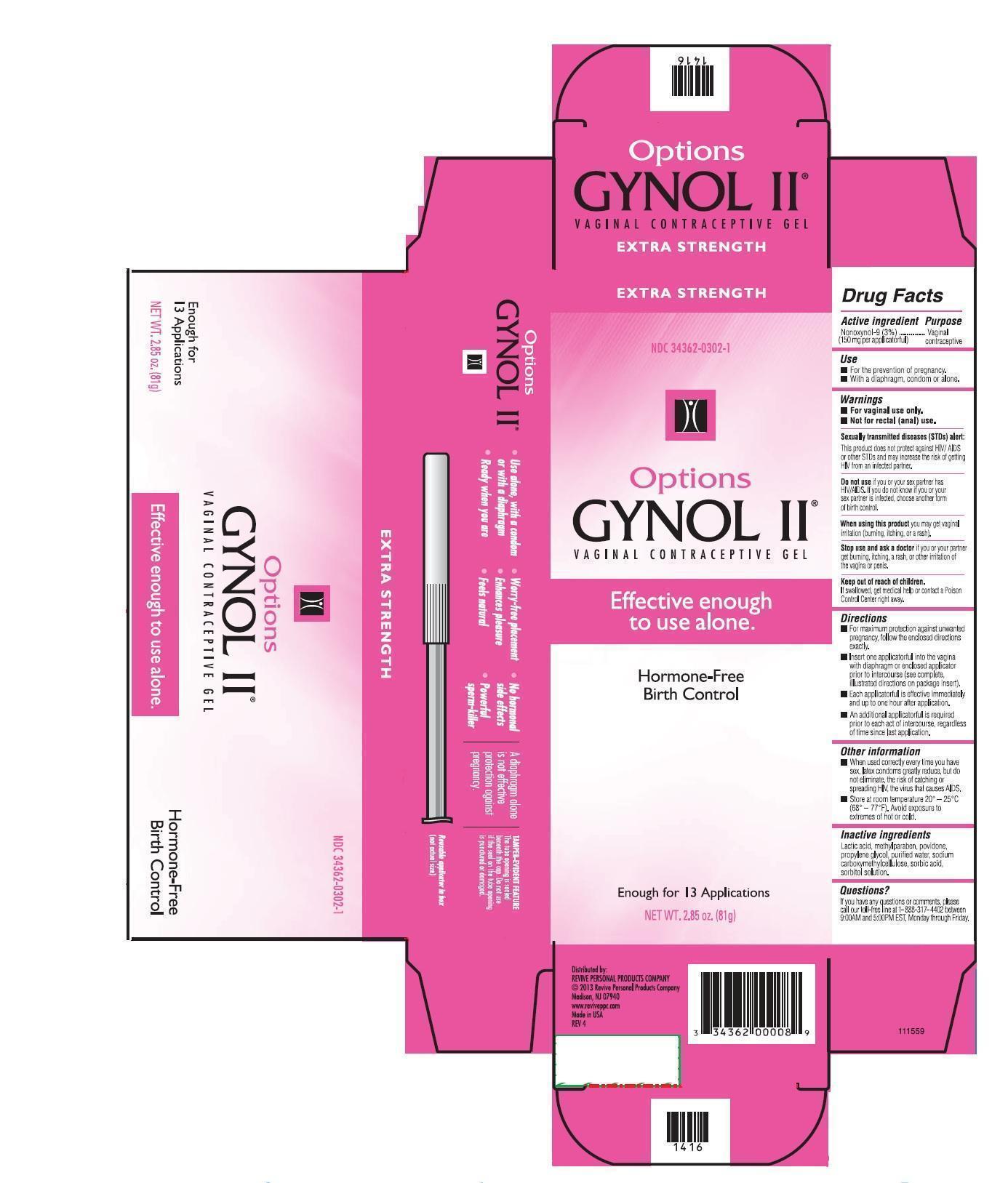
GYNOL II EXTRA STRENGTH- nonoxynol gel
Gynol II by
Drug Labeling and Warnings
Gynol II by is a Otc medication manufactured, distributed, or labeled by Caldwell Consumer Health LLC, Lornamead, DPT Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Uses
-
WARNINGS
For vaginal use only.
Not for rectal (anal) use.
Sexually transmitted diseases (STDs) alert:
This product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected partner.
Do not use if you or your sex partner has HIV/AIDS. If you do not know if your sex partner is infected, choose another form of birth control.
Stop use and ask a doctor if you or your partner gets burning, itching, a rash, or other irritation or the vagina or penis.
- Directions
- DOSAGE & ADMINISTRATION
- Other Information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GYNOL II EXTRA STRENGTH
nonoxynol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 34362-0302 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NONOXYNOL-9 (UNII: 48Q180SH9T) (NONOXYNOL-9 - UNII:48Q180SH9T) NONOXYNOL-9 3.0 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SORBIC ACID (UNII: X045WJ989B) METHYLPARABEN (UNII: A2I8C7HI9T) LACTIC ACID (UNII: 33X04XA5AT) POVIDONE K29/32 (UNII: 390RMW2PEQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 34362-0302-5 1 in 1 CARTON 1 NDC: 34362-0302-1 81 g in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2011 Labeler - Caldwell Consumer Health LLC (828558713) Registrant - Lornamead (126440440) Establishment Name Address ID/FEI Business Operations DPT Laboratories 832224526 manufacture(34362-0302) , pack(34362-0302)
Trademark Results [Gynol II]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GYNOL II 97755477 not registered Live/Pending |
HPSRX ENTERPRISES INC. 2023-01-16 |
 GYNOL II 85801818 4372344 Live/Registered |
Caldwell Consumer Health LLC 2012-12-13 |
 GYNOL II 73280655 1206690 Live/Registered |
Johnson & Johnson 1980-10-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.