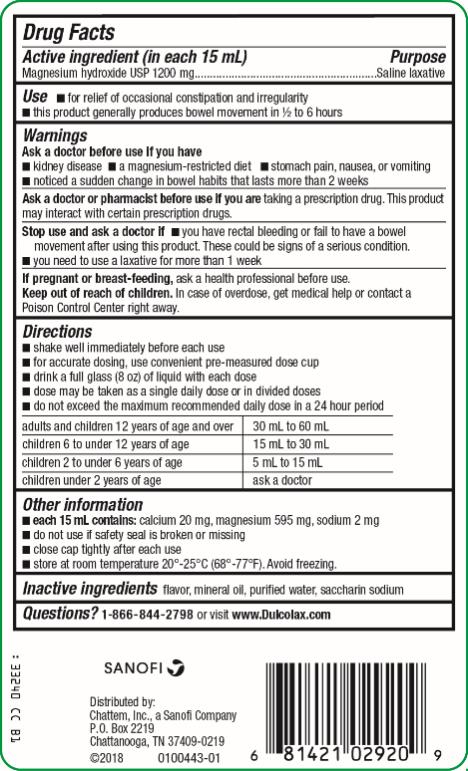
DULCOLAX LIQUID- magnesium hydroxide liquid
Dulcolax by
Drug Labeling and Warnings
Dulcolax by is a Otc medication manufactured, distributed, or labeled by Chattem, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 15 mL)
- Purpose
- Use
-
Warnings
Ask a doctor before use if you have
● kidney disease
● a magnesium-restricted diet
● stomach pain, nausea or vomiting
● noticed a sudden change in bowel habits that lasts more than 2 weeks
Ask a doctor or pharmacist before use if you are
taking a prescription drug. This product may interact with certain prescription drugs.
-
Directions
● shake well immediately before each use
● for accurate dosing, use convenient pre-measured dose cup
● drink a full glass (8 oz) of liquid with each dose
● dose may be taken as a single daily dose or in divided doses
● do not exceed the maximum recommended daily dose in a 24 hour period
adults and children 12 years of age and over 30 mL to 60 mL
children 6 to under 12 years of age 15 mL to 30 mL
children 2 to under 6 years of age 5 mL to 15 mL
children under 2 years of age ask a doctor
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DULCOLAX LIQUID
magnesium hydroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41167-0292 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 1200 mg in 15 mL Inactive Ingredients Ingredient Name Strength MINT (UNII: FV98Z8GITP) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41167-0292-0 354 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 03/01/2019 Labeler - Chattem, Inc. (003336013)
Trademark Results [Dulcolax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DULCOLAX 79043985 3505530 Live/Registered |
Sanofi-Aventis Deutschland GmbH 2007-08-16 |
 DULCOLAX 78157653 2861373 Dead/Cancelled |
Boehringer Ingelheim International GmbH 2002-08-26 |
 DULCOLAX 72042116 0671422 Live/Registered |
DR, KARL THOMAE GMBH 1957-12-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.