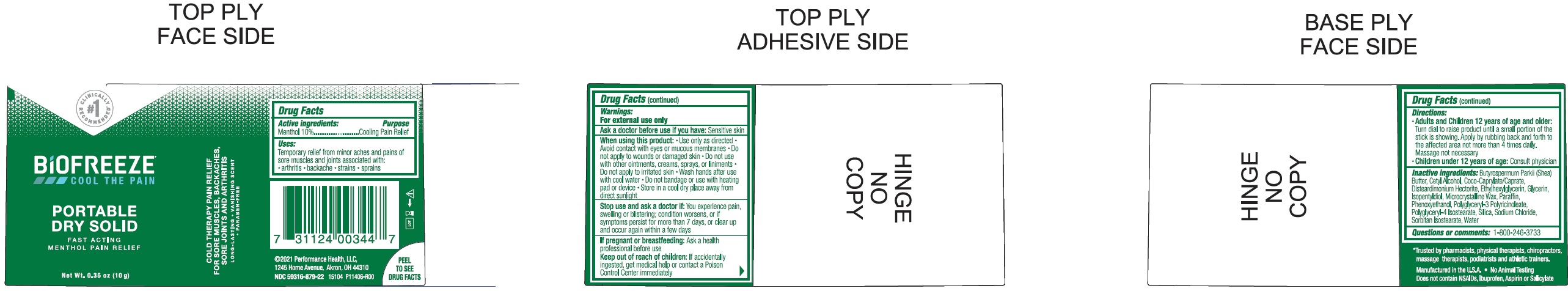
Biofreeze Portable Dry Solid
Biofreeze Portable Dry Solid by
Drug Labeling and Warnings
Biofreeze Portable Dry Solid by is a Otc medication manufactured, distributed, or labeled by Performance Health LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIOFREEZE PORTABLE DRY SOLID- menthol stick
Performance Health LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Biofreeze Portable Dry Solid
Uses:
Temporary relief from minor aches and pains of sore muscles and joints associated with: arthritis backache strains sprains
Warnings:
For external use only
When using this product:
Use only as directed Avoid contact with eyes or mucous membranes Do not apply to wounds or damaged skin Do not use with other ointments, creams, sprays, or liniments Do not apply to irritated skin Wash hands after use with cool water Do not bandage or use with heating pad or device Store in a cool dry place away from direct sunlight
Directions
Adults and Children 12 years of age and older: Turn dial to raise product until a small portion of the stick is showing. Apply by rubbing back and forth to the affected area not more than 4 times daily. Massage not necessary
Children under 12 years of age: Consult physician
Inactive Ingredients
Butyrospermum Parkii (Shea) Butter, Cetyl Alcohol, Coco-caprylate/Caprate, Disteardimonium Hectorite, Ethylhexylglycerin, Glycerin, Isopentyldiol, Wax, Paraffin, Phenoxyethanol, Polyglyceryl-3 Polyricinoleate, Polyglyceryl-4 Isostearate, Silica, Sodium Chloride, Sorbitan Isostearate, Water
| BIOFREEZE PORTABLE DRY SOLID
menthol stick |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Performance Health LLC (794324061) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.