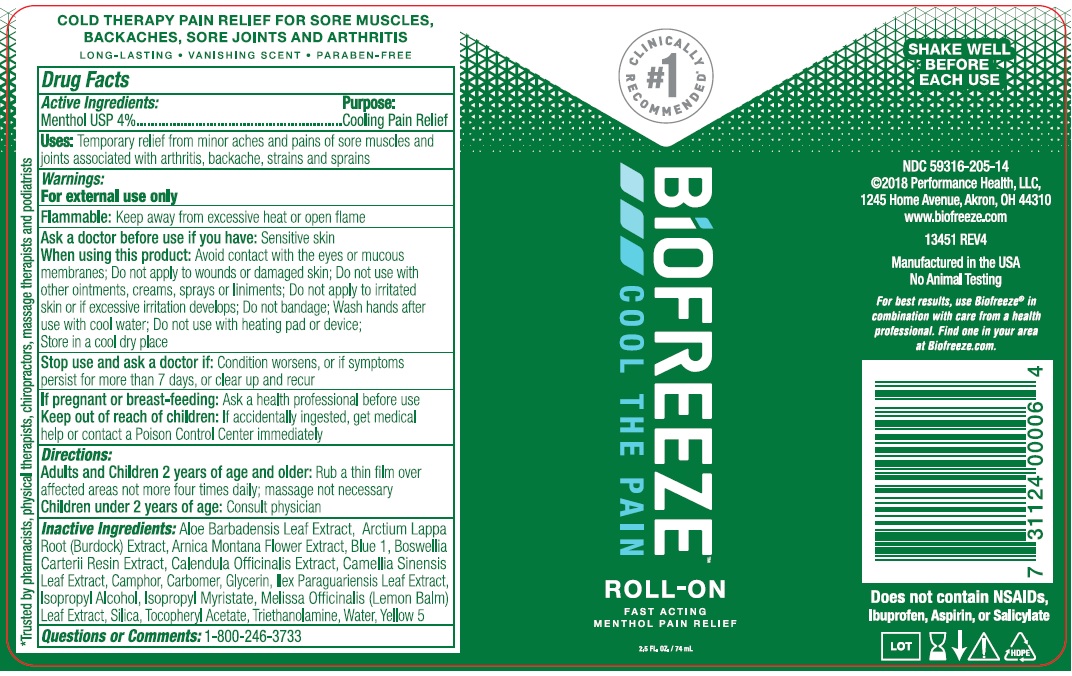
BIOFREEZE ROLL-ON- menthol gel
Biofreeze Roll-On by
Drug Labeling and Warnings
Biofreeze Roll-On by is a Otc medication manufactured, distributed, or labeled by RB Health (US) LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredients
- Purpose
- Uses:
-
Warnings:
For external use only
Flammable: Keep away from excessive heat or open flame
When using this product:
- Avoid contact with eyes or mucous membranes
- Do not apply to wounds or damaged skin.
- Do not use with other ointments, creams, sprays, or liniments.
- Do not apply to irritated skin or if excessive skin irritation develops.
- Do not bandage
- Wash hands after use with cool water
- Do not use with heating pad or device.
- Store in a cool dry place
- Directions
-
Inactive Ingredients:
Aloe Barbadensis Leaf Extract, Arnica Montana Flower Extract, Arctium Lappa Root (Burdock) Extract, Boswellia Carteril Resin Extract, Calendula Officinallis Extract, Carbomer, Camellia Sinensis (Green Tea) Leaf Extract, Camphor USP, Glycerin, Ilex Paraguariensis Leaf Extract, Isopropyl Alcohol, Ispropyl Myristate, Melissa Officinalis (Lemon Balm) Leaf Extract, Silicon Dioxide, Tocopheryl (Vitamin E) Acetate, Triethanolamine, Purified Water USP, Blue 1, Yellow 5
- Questions or Comments
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BIOFREEZE ROLL-ON
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59316-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) FRANKINCENSE (UNII: R9XLF1R1WM) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GLYCERIN (UNII: PDC6A3C0OX) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL ALCOHOL (UNII: ND2M416302) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59316-205-10 1 in 1 CARTON 01/17/2013 1 89 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC: 59316-205-14 74 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/17/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/17/2013 Labeler - Performance Health Inc. (794324061)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.