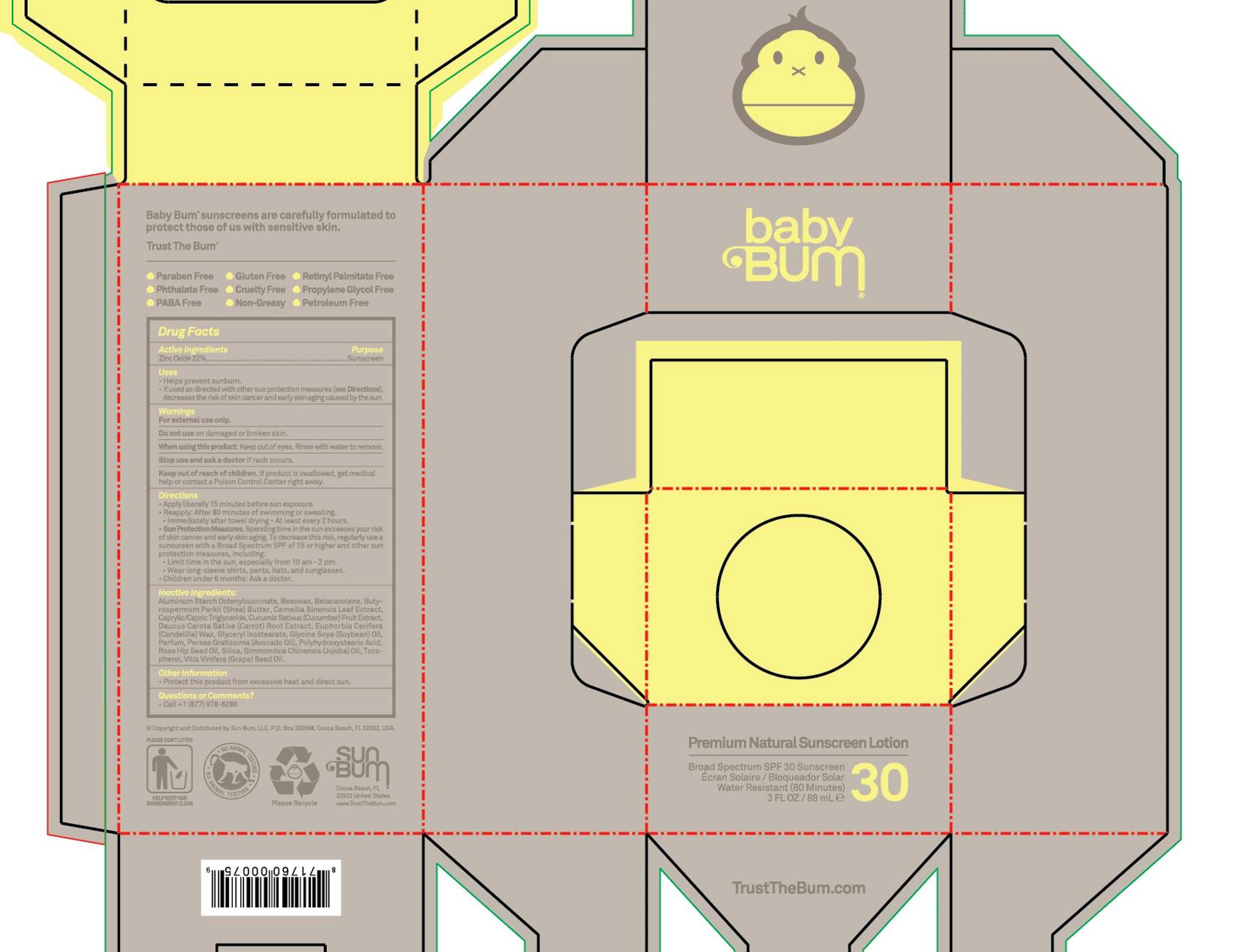
Premium Natural Sunscreen Lotion Baby Bum SPF 30
Premium Natural Sunscreen Loition by
Drug Labeling and Warnings
Premium Natural Sunscreen Loition by is a Otc medication manufactured, distributed, or labeled by Sun Bum LLC, Baxter Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PREMIUM NATURAL SUNSCREEN LOITION BABY BUM- zinc oxide cream
Sun Bum LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Premium Natural Sunscreen Lotion Baby Bum SPF 30
Uses
- Helps preven sunburn
- If used as directed with other sun protection measures (see Directions), decreasess the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Warnings
For external use only
Do not use on damaged or broken skin
When using this product: Keep out of eyes, Rinse with water to remove
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply: After 80 minutes of swimming or sweating
- Immediately after towel drying
- At least every 2 hours
- Sun Protection Measures. Spenidng time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures, including:
- Limit time in the sun, especially from 10 am - 2 pm.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
Inactive Ingredients:
Aluminum Startch Octenylsuccinate, Beeswax, Betacarotene, Butyrospermum Parkii (shea) Butter, Camellia Sinensis Leaf Extract, Caprylic/Capric Triglyceride, Cucumi Sativus (Cucumber) Fruit Extract, Daucus Carota Sativa (Carrot) Root Extract, Euphorbia Cerifera (Candelilla) Wax, Glyceryl Isostearate, Glycine Soya (Soybean) Oil, Parfum, Persea Gratissima (Avocado Oil), Polyhydroxystearic Acid, Rose Hip Seed Oi, Silica, Simmondsia Chinensis (Jojoba) Oil, Tocopherol, Vitis Vinifera (Grape) Seed Oil
| PREMIUM NATURAL SUNSCREEN LOITION
BABY BUM
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Sun Bum LLC (028642574) |
| Registrant - Sun Bum LLC (028642574) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Laboratories | 740537709 | manufacture(69039-104) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.