MONJUVI- tafasitamab-cxix injection, powder, lyophilized, for solution
MONJUVI by
Drug Labeling and Warnings
MONJUVI by is a Prescription medication manufactured, distributed, or labeled by Incyte Corporation, MILLMOUNT HEALTHCARE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MONJUVI safely and effectively. See full prescribing information for MONJUVI.
MONJUVI ®(tafasitamab-cxix) for injection, for intravenous use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
MONJUVI is a CD19-directed cytolytic antibody indicated in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT).
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). ( 1)
DOSAGE AND ADMINISTRATION
- Administer premedications prior to starting MONJUVI. ( 2.2)
- The recommended dosage of MONJUVI is 12 mg/kg as an intravenous infusion according to the following dosing schedule: (
2.1)
- Cycle 1: Days 1, 4, 8, 15 and 22 of the 28-day cycle.
- Cycles 2 and 3: Days 1, 8, 15 and 22 of each 28-day cycle.
- Cycle 4 and beyond: Days 1 and 15 of each 28-day cycle.
- Administer MONJUVI in combination with lenalidomide for a maximum of 12 cycles and then continue MONJUVI as monotherapy until disease progression or unacceptable toxicity. ( 2.1)
- See Full Prescribing Information for instructions on preparation and administration. ( 2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 200 mg of tafasitamab-cxix as lyophilized powder in single-dose vial for reconstitution.( 3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Infusion-Related Reactions: Monitor patients frequently during infusion. Interrupt or discontinue infusion based on severity. ( 2.3, 5.1)
- Myelosuppression: Monitor complete blood counts. Manage using dose modifications and growth factor support. Interrupt or discontinue MONJUVI based on severity. ( 2.3, 5.2)
- Infections: Bacterial, fungal and viral infections can occur during and following MONJUVI. Monitor patients for infections. ( 2.3, 5.3)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. ( 5.4)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) are neutropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, pyrexia, peripheral edema, respiratory tract infection, and decreased appetite. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact MORPHOSYS US INC. at 1-844-667-1992 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Premedications
2.3 Dosage Modifications for Adverse Reactions
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
5.2 Myelosuppression
5.3 Infections
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
MONJUVI, in combination with lenalidomide, is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT).
This indication is approved under accelerated approval based on overall response rate [see Clinical Studies (14)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of MONJUVI is 12 mg/kg based on actual body weight administered as an intravenous infusion according to the dosing schedule in Table 1.
Administer MONJUVI in combination with lenalidomide 25 mg for a maximum of 12 cycles, then continue MONJUVI as monotherapy until disease progression or unacceptable toxicity [see Clinical Studies (14)]. Refer to the lenalidomide prescribing information for lenalidomide dosage recommendations.
Table 1: MONJUVI Dosing Schedule Cycle * Dosing Schedule - * Each treatment cycle is 28-days.
Cycle 1 Days 1, 4, 8, 15 and 22 Cycles 2 and 3 Days 1, 8, 15 and 22 Cycle 4 and beyond Days 1 and 15 MONJUVI should be administered by a healthcare professional with immediate access to emergency equipment and appropriate medical support to manage infusion-related reactions (IRRs) [see Warnings and Precautions (5.1)] .
2.2 Recommended Premedications
Administer premedications 30 minutes to 2 hours prior to starting MONJUVI infusion to minimize infusion-related reactions [see Warnings and Precautions (5.1)]. Premedications may include acetaminophen, histamine H 1receptor antagonists, histamine H 2receptor antagonists, and/or glucocorticosteroids.
For patients not experiencing infusion-related reactions during the first 3 infusions, premedication is optional for subsequent infusions.
If a patient experiences an infusion-related reaction, administer premedications before each subsequent infusion.
2.3 Dosage Modifications for Adverse Reactions
The recommended dosage modifications for adverse reactions are summarized in Table 2.
Table 2: Dosage Modifications for Adverse Reactions Adverse Reaction Severity Dosage Modification Infusion-related reactions [see Warnings and Precautions (5.1)] Grade 2 (moderate) - Interrupt infusion immediately and manage signs and symptoms.
- Once signs and symptoms resolve or reduce to Grade 1, resume infusion at no more than 50% of the rate at which the reaction occurred. If the patient does not experience further reaction within 1 hour and vital signs are stable, the infusion rate may be increased every 30 minutes as tolerated to the rate at which the reaction occurred.
Grade 3 (severe) - Interrupt infusion immediately and manage signs and symptoms.
- Once signs and symptoms resolve or reduce to Grade 1, resume infusion at no more than 25% of the rate at which the reaction occurred. If the patient does not experience further reaction within 1 hour and vital signs are stable, the infusion rate may be increased every 30 minutes as tolerated to a maximum of 50% of the rate at which the reaction occurred.
- If after rechallenge the reaction returns, stop the infusion immediately.
Grade 4 (life-threatening) - Stop the infusion immediately and permanently discontinue MONJUVI.
Myelosuppression [see Warnings and Precautions (5.2)] Platelet count of 50,000/ mcL or less - Withhold MONJUVI and lenalidomide and monitor complete blood count (CBC) weekly until platelet count is 50,000/mcL or higher.
- Resume MONJUVI at the same dose and lenalidomide at a reduced dose. Refer to lenalidomide prescribing information for dosage modifications.
Neutrophil count of 1,000/ mcL or less for at least 7 days OR
Neutrophil count of 1,000/ mcL or less with an increase of body temperature to 100.4°F (38°C) or higher OR
Neutrophil count less than 500/mcL- Withhold MONJUVI and lenalidomide and monitor CBC weekly until neutrophil count is 1,000/ mcL or higher.
- Resume MONJUVI at the same dose and lenalidomide at a reduced dose. Refer to lenalidomide prescribing information for dosage modifications.
2.4 Preparation and Administration
Reconstitute and dilute MONJUVI prior to infusion.
Reconstitution
- Calculate the dose (mg) and determine the number of vials needed.
- Reconstitute each 200 mg MONJUVI vial with 5 mL Sterile Water for Injection, USP with the stream directed toward the wall of each vial to obtain a final concentration of 40 mg/mL tafasitamab-cxix.
- Gently swirl the vial(s) until completely dissolved. Do not shake or swirl vigorously. Complete dissolution may take up to 5 minutes.
- Visually inspect the reconstituted solution for particulate matter or discoloration. The reconstituted solution should appear as a colorless to slightly yellow solution. Discard the vial(s) if the solution is cloudy, discolored, or contains visible particles.
- Use the reconstituted MONJUVI solution immediately. If needed, store the reconstituted solution in the vial for a maximum of 12 hours either refrigerated at 36°F to 46°F (2°C to 8°C) or room temperature at 68°F to 77°F (20°C to 25°C) before dilution. Protect from light during storage.
Dilution
- Determine the volume (mL) of the 40 mg/mL reconstituted MONJUVI solution needed based on the required dose.
- Remove a volume equal to the required MONJUVI solution from a 250 mL 0.9% Sodium Chloride Injection, USP infusion bag and discard it.
- Withdraw the necessary amount of MONJUVI and slowly dilute in the infusion bag that contains the 0.9% Sodium Chloride Injection, USP to a final concentration of 2 mg/mL to 8 mg/mL. Discard any unused portion of MONJUVI remaining in the vial.
- Gently mix the intravenous bag by slowly inverting the bag. Do not shake. Visually inspect the infusion bag with the diluted MONJUVI infusion solution for particulate matter and discoloration prior to administration.
- If not used immediately, store the diluted MONJUVI infusion solution refrigerated for up to 18 hours at 36°F to 46°F (2°C to 8°C) and/or at room temperature for up to 12 hours at 68°F to 77°F (20°C to 25°C). The room temperature storage includes time for infusion. Protect from light during storage.
Do not shake or freeze the reconstituted or diluted infusion solutions.
Administration
- Administer MONJUVI as an intravenous infusion.
- For the first infusion, use an infusion rate of 70 mL/h for the first 30 minutes, then, increase the rate so that the infusion is administered within 1.5 to 2.5 hours.
- Administer all subsequent infusions within 1.5 to 2 hours.
- Infuse the entire contents of the bag containing MONJUVI.
- Do not co-administer other drugs through the same infusion line.
- No incompatibilities have been observed between MONJUVI with infusion containers made of polypropylene (PP), polyvinylchloride (PVC), polyethylene (PE), polyethylenterephthalate (PET), or glass and infusion sets made of polyurethane (PUR) or PVC.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
MONJUVI can cause infusion-related reactions [see Adverse Reactions (6.1)] . In L-MIND, infusion-related reactions occurred in 6% of the 81 patients. Eighty percent of infusion-related reactions occurred during cycle 1 or 2. Signs and symptoms included fever, chills, rash, flushing, dyspnea, and hypertension. These reactions were managed with temporary interruption of the infusion and/or with supportive medication.
Premedicate patients prior to starting MONJUVI infusion [see Dosage and Administration (2.2)] . Monitor patients frequently during infusion. Based on the severity of the infusion-related reaction, interrupt or discontinue MONJUVI [see Dosage and Administration (2.3)] . Institute appropriate medical management.
5.2 Myelosuppression
MONJUVI can cause serious or severe myelosuppression, including neutropenia, thrombocytopenia, and anemia [see Adverse Reactions (6.1)] . In L-MIND, Grade 3 neutropenia occurred in 25% of patients, thrombocytopenia in 12%, and anemia in 7%. Grade 4 neutropenia occurred in 25% and thrombocytopenia in 6%. Neutropenia led to treatment discontinuation in 3.7% of patients.
Monitor CBC prior to administration of each treatment cycle and throughout treatment. Monitor patients with neutropenia for signs of infection. Consider granulocyte colony-stimulating factor administration. Withhold MONJUVI based on the severity of the adverse reaction [see Dosage and Administration (2.3)]. Refer to the lenalidomide prescribing information for dosage modifications.
5.3 Infections
Fatal and serious infections, including opportunistic infections, occurred in patients during treatment with MONJUVI and following the last dose [see Adverse Reactions (6.1)].
In L-MIND, 73% of the 81 patients developed an infection. The most frequent infections were respiratory tract infection (24%), urinary tract infection (17%), bronchitis (16%), nasopharyngitis (10%) and pneumonia (10%). Grade 3 or higher infection occurred in 30% of the 81 patients. The most frequent grade 3 or higher infection was pneumonia (7%). Infection-related deaths were reported in 2.5% of the 81 patients.
Monitor patients for signs and symptoms of infection and manage infections as appropriate.
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, MONJUVI may cause fetal B-cell depletion when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with MONJUVI and for at least 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)] .
MONJUVI is initially administered in combination with lenalidomide. The combination of MONJUVI with lenalidomide is contraindicated in pregnant women because lenalidomide can cause birth defects and death of the unborn child. Refer to the lenalidomide prescribing information on use during pregnancy.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infusion-related reactions [see Warnings and Precautions (5.1)]
- Myelosuppression [see Warnings and Precautions (5.2)]
- Infections [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in other clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed or Refractory Diffuse Large B-Cell Lymphoma
The safety of MONJUVI was evaluated in L-MIND [see Clinical Studies (14)]. Patients (n=81) received MONJUVI 12 mg/kg intravenously in combination with lenalidomide for a maximum of 12 cycles, followed by MONJUVI as monotherapy until disease progression or unacceptable toxicity as follows:
- Cycle 1: Days 1, 4, 8, 15 and 22 of the 28-day cycle;
- Cycles 2 and 3: Days 1, 8, 15 and 22 of each 28-day cycle;
- Cycles 4 and beyond: Days 1 and 15 of each 28-day cycle.
Among patients who received MONJUVI, 57% were exposed for 6 months or longer, 42% were exposed for greater than one year, and 24% were exposed for greater than two years.
Serious adverse reactions occurred in 52% of patients who received MONJUVI. Serious adverse reactions in ≥6% of patients included infections (26%), including pneumonia (7%), and febrile neutropenia (6%). Fatal adverse reactions occurred in 5% of patients who received MONJUVI, including cerebrovascular accident (1.2%), respiratory failure (1.2%), progressive multifocal leukoencephalopathy (1.2%) and sudden death (1.2%).
Permanent discontinuation of MONJUVI or lenalidomide due to an adverse reaction occurred in 25% of patients and permanent discontinuation of MONJUVI due to an adverse reaction occurred in 15%. The most frequent adverse reactions which resulted in permanent discontinuation of MONJUVI were infections (5%), nervous system disorders (2.5%), respiratory, thoracic and mediastinal disorders (2.5%).
Dosage interruptions of MONJUVI or lenalidomide due to an adverse reaction occurred in 69% of patients and dosage interruption of MONJUVI due to an adverse reaction occurred in 65%. The most frequent adverse reactions which required a dosage interruption of MONJUVI were blood and lymphatic system disorders (41%), and infections (27%).
The most common adverse reactions (≥ 20%) were neutropenia, fatigue, anemia, diarrhea, thrombocytopenia, cough, pyrexia, peripheral edema, respiratory tract infection, and decreased appetite.
Table 3 summarizes the adverse reactions in L-MIND.
Table 3: Adverse Reactions (≥10%) in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma Who Received MONJUVI in L-MIND Adverse Reaction MONJUVI
(N=81)All Grades
(%)Grade 3 or 4
(%)- * Fatigue includes asthenia and fatigue
- † Abdominal pain includes abdominal pain, abdominal pain lower, and abdominal pain upper
- ‡ Respiratory tract infection includes: lower respiratory tract infection, upper respiratory tract infection, respiratory tract infection
- § Urinary tract infection includes: urinary tract infection, Escherichia urinary tract infection, urinary tract infection bacterial, urinary tract infection enterococcal
- ¶ Rash includes rash, rash maculo-papular, rash pruritic, rash erythematous , rash pustular
Blood and lymphatic system disorders Neutropenia 51 49 Anemia 36 7 Thrombocytopenia 31 17 Febrile neutropenia 12 12 General disorders and administration site conditions Fatigue * 38 3.7 Pyrexia 24 1.2 Peripheral edema 24 0 Gastrointestinal disorders Diarrhea 36 1.2 Constipation 17 0 Abdominal pain † 15 1.2 Nausea 15 0 Vomiting 15 0 Respiratory, thoracic and mediastinal disorders Cough 26 1.2 Dyspnea 12 1.2 Infections Respiratory tract infection ‡ 24 4.9 Urinary tract infection § 17 4.9 Bronchitis 16 1.2 Metabolism and nutrition disorders Decreased appetite 22 0 Hypokalemia 19 6 Musculoskeletal and connective tissue disorders Back pain 19 2.5 Muscle spasms 15 0 Skin and subcutaneous tissue disorders Rash ¶ 15 2.5 Pruritus 10 1.2 Clinically relevant adverse reactions in <10% of patients who received MONJUVI were:
- Blood and lymphatic system disorders: lymphopenia (6%)
- General disorders and administration site conditions: infusion-related reaction (6%)
- Infections: sepsis (4.9%)
- Investigations: weight decreased (4.9%)
- Musculoskeletal and connective tissue disorders: arthralgia (9%), pain in extremity (9%), musculoskeletal pain (2.5%)
- Neoplasms benign, malignant and unspecified: basal cell carcinoma (1.2%)
- Nervous system disorders:headache (9%), paresthesia (7%), dysgeusia (6%)
- Respiratory, thoracic and mediastinal disorders:nasal congestion (4.9%), exacerbation of chronic obstructive pulmonary disease (1.2%)
- Skin and subcutaneous tissue disorders:erythema (4.9%), alopecia (2.5%), hyperhidrosis (2.5%)
Table 4 summarizes the laboratory abnormalities in L-MIND.
Table 4: Select Laboratory Abnormalities (>20%) Worsening from Baseline in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma Who Received MONJUVI in L-MIND Laboratory Abnormality MONJUVI * All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate was 74 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Glucose increased 49 5 Calcium decreased 47 1.4 Gamma glutamyl transferase increased 34 5 Albumin decreased 26 0 Magnesium decreased 22 0 Urate increased 20 7 Phosphate decreased 20 5 Creatinine increased 20 1.4 Aspartate aminotransferase increased 20 0 Coagulation Activated partial thromboplastin time increased 46 4.1 6.2 Immunogenicity
As with all therapeutic proteins, there is the potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assays. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other tafasitamab products may be misleading.
Overall, no treatment-emergent or treatment-boosted anti-tafasitamab antibodies were observed. No clinically meaningful differences in the pharmacokinetics, efficacy, or safety profile of tafasitamab-cxix were observed in 2.5% of 81 patients with relapsed or refractory DLBCL with pre-existing anti-tafasitamab antibodies in L-MIND.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, MONJUVI may cause fetal B-cell depletion when administered to a pregnant woman [see Clinical Pharmacology (12.1)] . There are no available data on MONJUVI use in pregnant women to evaluate for a drug-associated risk. Animal reproductive toxicity studies have not been conducted with tafasitamab-cxix.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
MONJUVI is administered in combination with lenalidomide for up to 12 cycles. Lenalidomide can cause embryo-fetal harm and is contraindicated for use in pregnancy. Refer to the lenalidomide prescribing information for additional information. Lenalidomide is only available through a REMS program.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Immunoglobulin G (IgG) monoclonal antibodies are transferred across the placenta. Based on its mechanism of action, MONJUVI may cause depletion of fetal CD19 positive immune cells. Defer administering live vaccines to neonates and infants exposed to tafasitamab-cxix in utero until a hematology evaluation is completed.
8.2 Lactation
Risk Summary
There are no data on the presence of tafasitamab-cxix in human milk or the effects on the breastfed child or milk production. Maternal immunoglobulin G is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to MONJUVI are unknown. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with MONJUVI and for at least 3 months after the last dose. Refer to lenalidomide prescribing information for additional information.
8.3 Females and Males of Reproductive Potential
MONJUVI can cause fetal B-cell depletion when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Refer to the prescribing information for lenalidomide for pregnancy testing requirements prior to initiating the combination of MONJUVI with lenalidomide.
8.4 Pediatric Use
The safety and effectiveness of MONJUVI in pediatric patients have not been established.
8.5 Geriatric Use
Among 81 patients who received MONJUVI and lenalidomide in L-MIND, 72% were 65 years and older, while 38% were 75 years and older. Clinical studies of MONJUVI did not include sufficient numbers of patients aged 65 and older to determine whether effectiveness differs compared to that of younger subjects. Patients 65 years and older had more serious adverse reactions (57%) than younger patients (39%).
-
11 DESCRIPTION
Tafasitamab-cxix is a humanized CD19-directed cytolytic monoclonal antibody that contains an IgG1/2 hybrid Fc-domain with 2 amino acid substitutions to modify the Fc-mediated functions of the antibody. It is produced by recombinant DNA technology in mammalian cells (Chinese hamster ovary). Tafasitamab-cxix has a molecular weight of approximately 150 kDa.
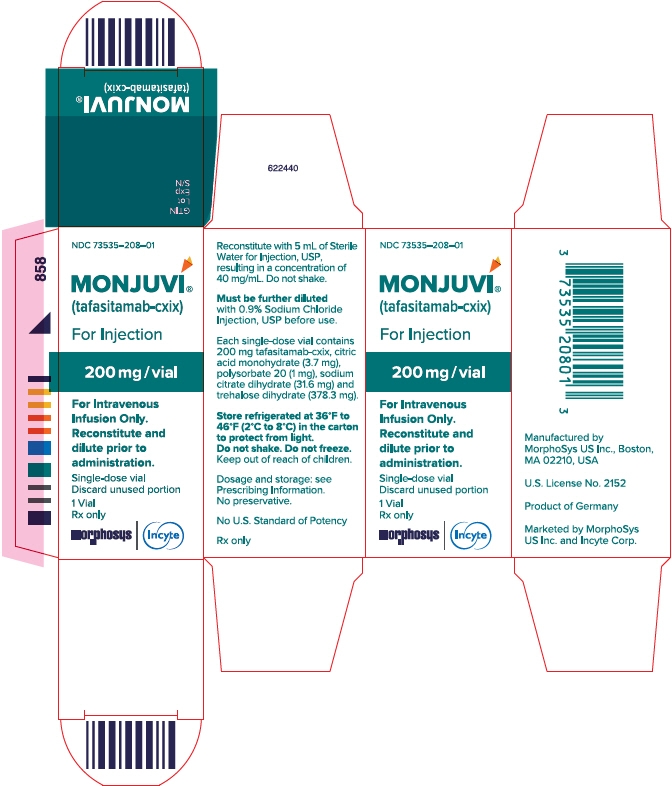
MONJUVI (tafasitamab-cxix) for injection is supplied as a sterile, preservative-free, white to slightly yellowish lyophilized powder in a single-dose vial for intravenous use after reconstitution and further dilution. After reconstitution with 5 mL of Sterile Water for Injection, USP, the resulting concentration is 40 mg/mL with a pH of 6.0. Each single-dose vial contains 200 mg tafasitamab-cxix, citric acid monohydrate (3.7 mg), polysorbate 20 (1 mg), sodium citrate dihydrate (31.6 mg) and trehalose dihydrate (378.3 mg).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tafasitamab-cxix is an Fc-modified monoclonal antibody that binds to CD19 antigen expressed on the surface of pre-B and mature B lymphocytes and on several B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL).
Upon binding to CD19, tafasitamab-cxix mediates B-cell lysis through apoptosis and immune effector mechanisms, including antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
In studies conducted in vitro in DLBCL tumor cells, tafasitamab-cxix in combination with lenalidomide resulted in increased ADCC activity compared to tafasitamab-cxix or lenalidomide alone.
12.2 Pharmacodynamics
Tafasitamab-cxix reduced peripheral blood B cell counts by 97% after eight days of treatment in patients with relapsed or refractory DLBCL. Nadir, with a reduction of 100%, was reached within 16 weeks of treatment.
12.3 Pharmacokinetics
Mean trough concentrations (± standard deviation) were 179 (± 53) μg/mL following administration of MONJUVI at 12 mg/kg on Days 1, 8, 15, and 22 in Cycle 1-3 (plus an additional dose on Cycle 1 Day 4), and 153 (± 68) μg/mL following administration of MONJUVI at 12 mg/kg on Days 1 and 15 from Cycle 4 onwards. Overall maximum tafasitamab-cxix serum concentrations were 483 (±109) μg/mL.
Elimination
The clearance of tafasitamab-cxix was 0.41 L/day (CV: 32%) and terminal elimination half-life was 17 days (95% CI: 15, 18 days).
Specific Populations
Bodyweight (40 to 163 kg) has a significant effect on the pharmacokinetics of tafasitamab-cxix, with higher clearance and volume of distribution expected with higher body weight. No clinically meaningful differences in the pharmacokinetics of tafasitamab-cxix were observed based on age (16 to 90 years), sex, mild to moderate renal impairment (CLcr 30-89 mL/min estimated by the Cockcroft-Gault equation), and mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1 to 1.5 times ULN and any AST). The effect of severe renal impairment to end-stage renal disease (CLcr < 30 mL/min), moderate to severe hepatic impairment (total bilirubin > 1.5 times ULN and any AST), and race/ethnicity on tafasitamab-cxix pharmacokinetics is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and genotoxicity studies have not been conducted with tafasitamab-cxix.
Fertility studies have not been conducted with tafasitamab-cxix.
In the 13-week repeat-dose general toxicity study in cynomolgus monkeys, no adverse effects on male and female reproductive organs were observed up to the highest dose tested, 100 mg/kg/week (approximately 9 times the human exposure based on AUC at the clinical dose of 12 mg/kg/week).
-
14 CLINICAL STUDIES
The efficacy of MONJUVI in combination with lenalidomide followed by MONJUVI as monotherapy was evaluated in L-MIND, an open-label, multicenter, single arm trial (NCT02399085). Eligible patients had relapsed or refractory DLBCL after 1 to 3 prior systemic therapies, including a CD20-directed cytolytic antibody, and were not candidates for high dose chemotherapy (HDC) followed by autologous stem cell transplantation (ASCT). Patients received MONJUVI 12 mg/kg intravenously in combination with lenalidomide (25 mg orally on Days 1 to 21 of each 28-day cycle) for a maximum of 12 cycles, followed by MONJUVI as monotherapy until disease progression or unacceptable toxicity as follows:
- Cycle 1: Days 1, 4, 8, 15 and 22 of the 28-day cycle;
- Cycles 2 and 3: Days 1, 8, 15 and 22 of each 28-day cycle;
- Cycles 4 and beyond: Days 1 and 15 of each 28-day cycle.
Of the 71 patients with DLBCL confirmed by central laboratory who received the combination therapy, the median age was 71 years (range: 41 to 86 years); 55% were males, and 100% had received a prior CD20-containing therapy. Race was collected in 92% of patients; of these, 95% were White, and 3% were Asian. The median number of prior therapies was two; 49% had one prior line of treatment, and 51% had 2 to 4 prior lines. Thirty-two patients (45%) were refractory to their last prior therapy and 30 (42%) were refractory to rituximab. Nine patients (13%) had received prior ASCT. The primary reasons patients were not candidates for ASCT included age (47%), refractoriness to salvage chemotherapy (27%), comorbidities (13%) and refusal of high dose chemotherapy/ASCT (13%).
Efficacy was established based on best overall response rate, defined as the proportion of complete and partial responders, and duration of response, as assessed by an Independent Review Committee using the International Working Group Response Criteria (Cheson, 2007). Results are summarized in Table 5.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Infusion-Related Reactions
Advise patients to contact their healthcare provider if they experience signs and symptoms of infusion-related reactions [see Warnings and Precautions (5.1)] .
Myelosuppression
Inform patients about the risk of myelosuppression. Advise patients to immediately contact their healthcare provider for a fever of 100.4°F (38°C) or greater or signs or symptoms of bruising or bleeding. Advise patients of the need for periodic monitoring of blood counts [see Warnings and Precautions (5.2)] .
Infections
Inform patients about the risk of infections. Advise patients to immediately contact their healthcare provider for a fever of 100.4°F (38°C) or greater or signs or symptoms of infection [see Warnings and Precautions (5.3)] .
Embryo-Fetal Toxicity
- Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Population (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with MONJUVI and for at least 3 months after the last dose [see Use in Specific Populations (8.3)] .
- Advise patients that lenalidomide has the potential to cause fetal harm and has specific requirements regarding contraception, pregnancy testing, blood and sperm donation, and transmission in sperm. Lenalidomide is only available through a REMS program [see Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with MONJUVI and for at least 3 months after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: JUN 2021 PATIENT INFORMATION
MONJUVI ®(mon-JOO-vee)
(tafasitamab-cxix)
for injectionWhat is MONJUVI?
MONJUVI is a prescription medicine given with lenalidomide to treat adults with certain types of diffuse large B-cell lymphoma (DLBCL) that has come back (relapsed) or that did not respond to previous treatment (refractory) and who cannot receive a stem cell transplant.
It is not known if MONJUVI is safe and effective in children.Before you receive MONJUVI, tell your healthcare provider about all of your medical conditions, including if you: - have an active infection or have had one recently.
- are pregnant or plan to become pregnant. MONJUVI may harm your unborn baby. You should not become pregnant during treatment with MONJUVI. Do not receive treatment with MONJUVI in combination with lenalidomide if you are pregnant because lenalidomide can cause birth defects and death of your unborn baby.
- You should use an effective method of birth control (contraception) during treatment and for at least 3 months after your last dose of MONJUVI.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with MONJUVI.
- are breastfeeding or plan to breastfeed. It is not known if MONJUVI passes into your breastmilk. Do not breastfeed during treatment and for at least 3 months after your last dose of MONJUVI.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.How will I receive MONJUVI? - MONJUVI will be given to you by your healthcare provider as an intravenous (IV) infusion into one of your veins.
- Your healthcare provider will give you medicines before each infusion to decrease your chance of infusion reactions. If you do not have any reactions, your healthcare provider may decide that you do not need these medicines with later infusions.
- Each treatment cycle of MONJUVI lasts for 28 days.
- Your healthcare provider may need to delay or completely stop treatment with MONJUVI if you have severe side effects.
- Your healthcare provider will decide how many treatments you need.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of MONJUVI?
MONJUVI may cause serious side effects, including:- Infusion reactions.Your healthcare provider will monitor you for infusion reactions during your infusion of MONJUVI. Tell your healthcare provider right away if you get fever, chills, flushing, headache, or shortness of breath during an infusion of MONJUVI.
- Low blood cell counts(platelets, red blood cells, and white blood cells). Low blood cell counts are common with MONJUVI, but can also be serious or severe. Your healthcare provider will monitor your blood counts during treatment with MONJUVI. Tell your healthcare provider right away if you get a fever of 100.4°F (38°C) or above, or any bruising or bleeding.
- Infections.Serious infections, including infections that can cause death, have happened in people during treatment with MONJUVI and after the last dose. Tell your healthcare provider right away if you get a fever of 100.4°F (38°C) or above, or develop any signs or symptoms of an infection.
- feeling tired or weak
- diarrhea
- cough
- fever
- swelling of lower legs or hands
- respiratory tract infection
- decreased appetite
These are not all the possible side effects of MONJUVI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of MONJUVI.
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information. If you would like more information about MONJUVI, talk with your healthcare provider. You can ask your healthcare provider for information about MONJUVI that is written for health professionals.What are the ingredients in MONJUVI?
Active ingredient:tafasitamab-cxix.
Inactive ingredients:citric acid monohydrate, polysorbate 20, sodium citrate dihydrate, and trehalose dihydrate.
Manufactured by: MORPHOSYS US INC., Boston, MA 02210, U.S. License No. 2152,
Marketed by: MORPHOSYS US INC. and INCYTE Corporation.
MONJUVI is a registered trademark of MorphoSys AG. © 2020 MorphoSys AG. All rights reserved.
For more information call MorphoSys US Inc., at 1-844-667-1992 or go to www.MONJUVI.com. - PRINCIPAL DISPLAY PANEL - 200 mg Vial Carton
-
INGREDIENTS AND APPEARANCE
MONJUVI
tafasitamab-cxix injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73535-208 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAFASITAMAB (UNII: QQA9MLH692) (TAFASITAMAB - UNII:QQA9MLH692) TAFASITAMAB 200 mg in 5 mL Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 31.6 mg in 5 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 3.7 mg in 5 mL TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 378.3 mg in 5 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 1 mg in 5 mL Product Characteristics Color white (white to slightly yellowish lyophilized powder) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73535-208-01 1 in 1 CARTON 08/05/2020 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761163 08/05/2020 Labeler - Incyte Corporation (556967347)
Trademark Results [MONJUVI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MONJUVI 88682860 not registered Live/Pending |
MorphoSys AG 2019-11-06 |
 MONJUVI 88664626 not registered Live/Pending |
MorphoSys AG 2019-10-22 |
 MONJUVI 88074194 not registered Live/Pending |
MorphoSys AG 2018-08-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.