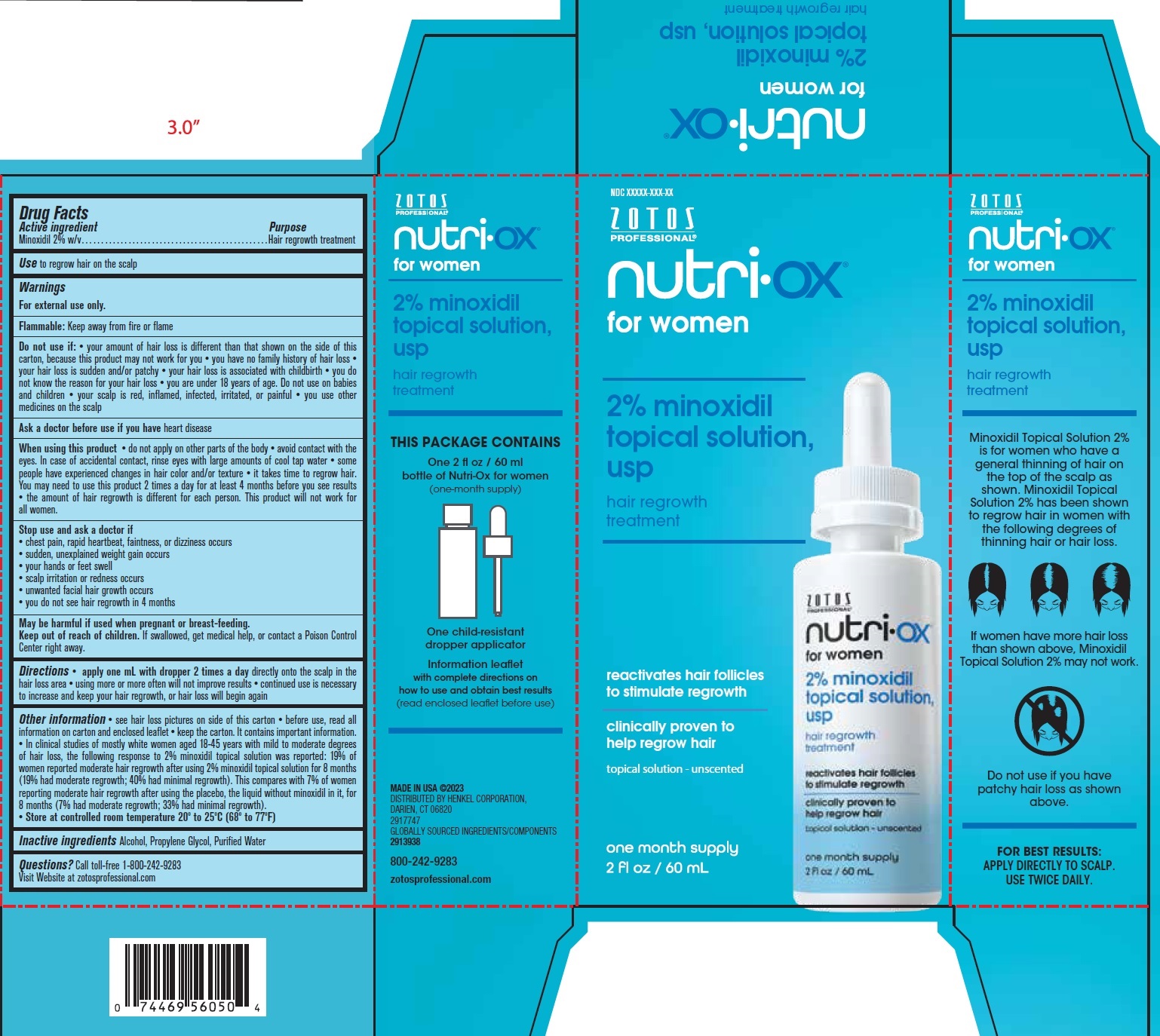
ZOTOS PROFESSIONAL Nutri for Women 2% Minoxidil Topical Solution
ZOTOS PROFESSIONAL Nutri for Women 2% Minoxidil Topical Solution by
Drug Labeling and Warnings
ZOTOS PROFESSIONAL Nutri for Women 2% Minoxidil Topical Solution by is a Otc medication manufactured, distributed, or labeled by HENKEL US OPERATIONS CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZOTOS PROFESSIONAL NUTRI FOR WOMEN 2% MINOXIDIL TOPICAL SOLUTION- minoxidil solution
HENKEL US OPERATIONS CORPORATION
----------
ZOTOS PROFESSIONAL Nutri for Women 2% Minoxidil Topical Solution
Warnings
For external use only.
Flammable: Keep away from fire or flame
Do not use if:
your amount of hair loss is different than that shown on the side of this carton, because this product may not work for you you have no family history of hair loss your hair loss is sudden and/or patchy your hair loss is associated with childbirth you do not know the reason for your hair loss you are under 18 years of age. Do not use on babies and children your scalp is red, inflamed, infected, irritated, or painful you use other medicines on the scalp
When using this product
do not apply on other parts of the body avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water some people have experienced changes in hair color and/or texture it takes time to regrow hair. You may need to use this product 2 times a day for at least 4 months before you see results the amount of hair regrowth is different for each person. This product will not work for all women.
Directions
apply one mL with dropper 2 times a day directly onto the scalp in the hair loss area using more or more often will not improve results continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again
Other information
see hair loss pictures on side of this carton before use, read all information on carton and enclosed leaflet keep the carton. It contains important information. In clinical studies of mostly white women aged 18-45 years with mild to moderate degrees of hair loss, the following response to 2% minoxidil topical solution was reported: 19% of women reported moderate hair regrowth after using 2% minoxidil topical solution for 8 months (19% had moderate regrowth; 40% had minimal regrowth). This compares with 7% of women reporting moderate hair regrowth after using the placebo, the liquid without minoxidil in it, for 8 months (7% had moderate regrowth; 33% had minimal regrowth).
Store at controlled room temperature 20º to 25ºC (68º to 77ºF)
| ZOTOS PROFESSIONAL NUTRI FOR WOMEN 2% MINOXIDIL TOPICAL SOLUTION
minoxidil solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HENKEL US OPERATIONS CORPORATION (966706145) |