amekina Instant Hand Sanitizer Gel 6047 Drug Facts and Label
amekina Instant Hand Sanitizer Gel by
Drug Labeling and Warnings
amekina Instant Hand Sanitizer Gel by is a Otc medication manufactured, distributed, or labeled by Angelini Pharma Inc., ABC Compounding Co., Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
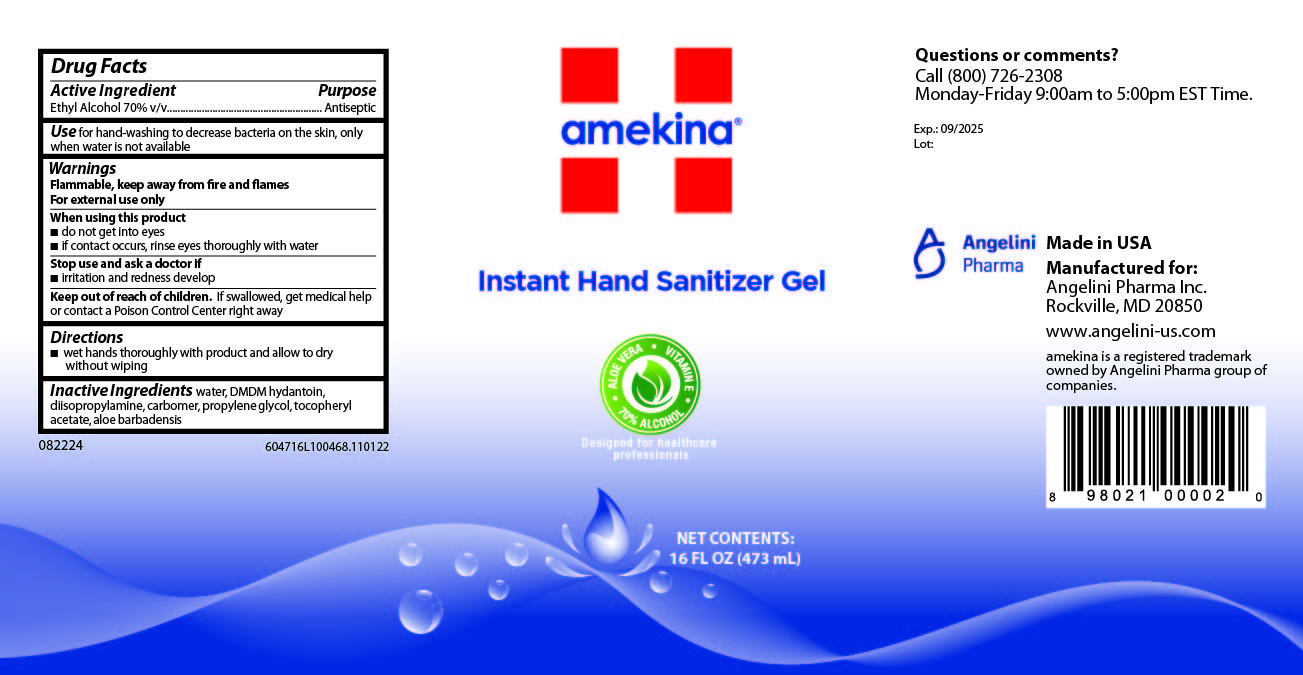
AMEKINA INSTANT HAND SANITIZER GEL- alcohol gel
Angelini Pharma Inc.
----------
amekina Instant Hand Sanitizer Gel 6047 Drug Facts and Label
Drug Facts Box OTC-Indications & Usage Section
for hand-washing to decrease bacteria on the skin, only when water is not available
Drug Facts Box OTC-When Using Section
do not get into eyes
if contact occurs, rinse eyes thoroughly with water
Drug Facts Box OTC-Keep Out of Reach of Children Section
if swallowed, get medical help or contact a Poison Control Center right away
Drug Facts Box OTC-Dosage & Administration Section
wet hands thoroughly with product and allow to dry without wiping
| AMEKINA INSTANT HAND SANITIZER GEL
alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Angelini Pharma Inc. (078843940) |
| Registrant - ABC Compounding Co., Inc. (003284353) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ABC Compounding Co., Inc. | 003284353 | manufacture(43595-122) | |
Revised: 1/2025
Document Id: 2cdbc5ab-33bf-b2d0-e063-6294a90af015
Set id: ec927c88-05ad-b1d9-e053-2a95a90a7dce
Version: 2
Effective Time: 20250129
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 Hand Sanitizer Gel.jpg
Hand Sanitizer Gel.jpg