CARDIOVASCULAR PROCEDURE KIT- kit
Cardiovascular Procedure Kit by
Drug Labeling and Warnings
Cardiovascular Procedure Kit by is a Other medication manufactured, distributed, or labeled by Centurion Medical Products, Hospira. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DESCRIPTION
- Cardiovascular Kit Primary Label
- Lidocaine Syringe Label
-
INGREDIENTS AND APPEARANCE
CARDIOVASCULAR PROCEDURE KIT
cardiovascular procedure kit kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:24840-1105 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:24840-1105-2 10 in 1 CASE 1 NHRIC:24840-1105-1 1 in 1 PACKAGE, COMBINATION Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, PLASTIC 5 mL Part 1 of 1 LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride injection, solutionProduct Information Item Code (Source) NDC: 0409-9137 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-9137-05 5 mL in 1 SYRINGE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040302 11/29/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device OEZ 01/01/2012 Labeler - Centurion Medical Products (017246562) Establishment Name Address ID/FEI Business Operations Centurion Medical Products 017246562 manufacture, repack Establishment Name Address ID/FEI Business Operations Centurion Medical Products 148522279 manufacture, repack Establishment Name Address ID/FEI Business Operations Centurion Medical Products 626660810 manufacture, repack Establishment Name Address ID/FEI Business Operations Hospira 093132819 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 MM1
MM1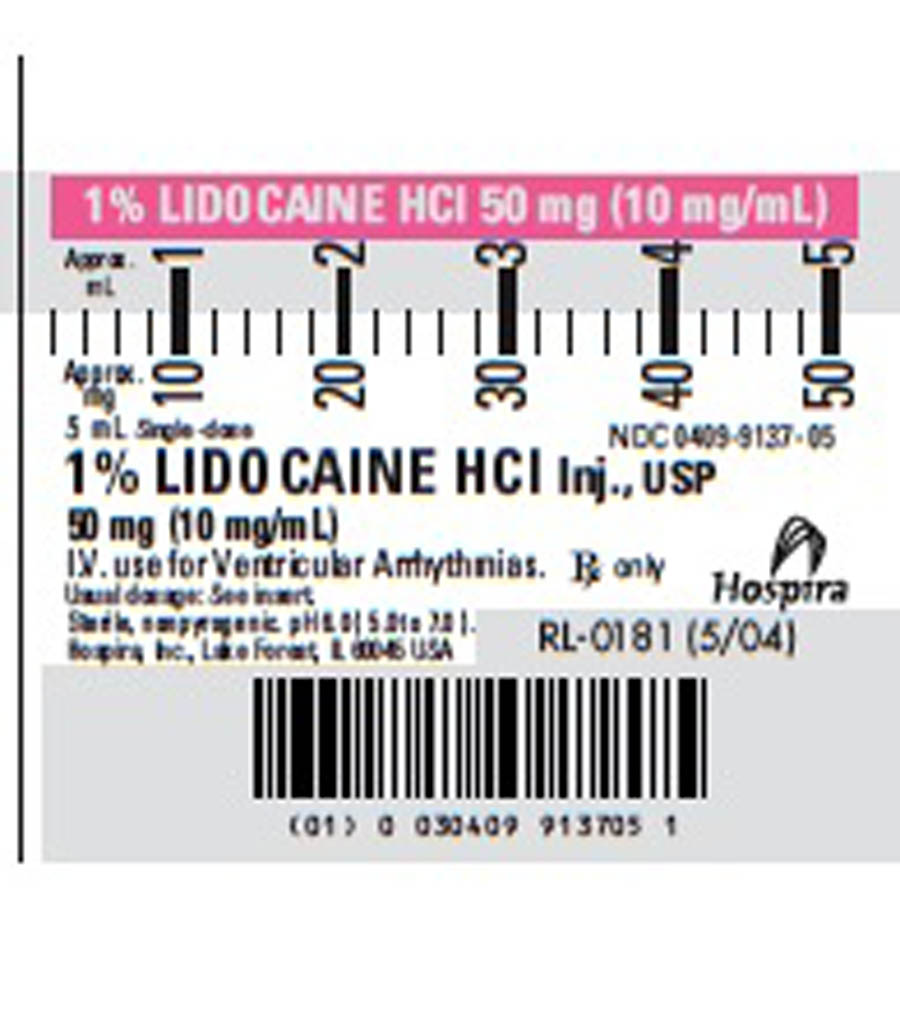 MM1
MM1