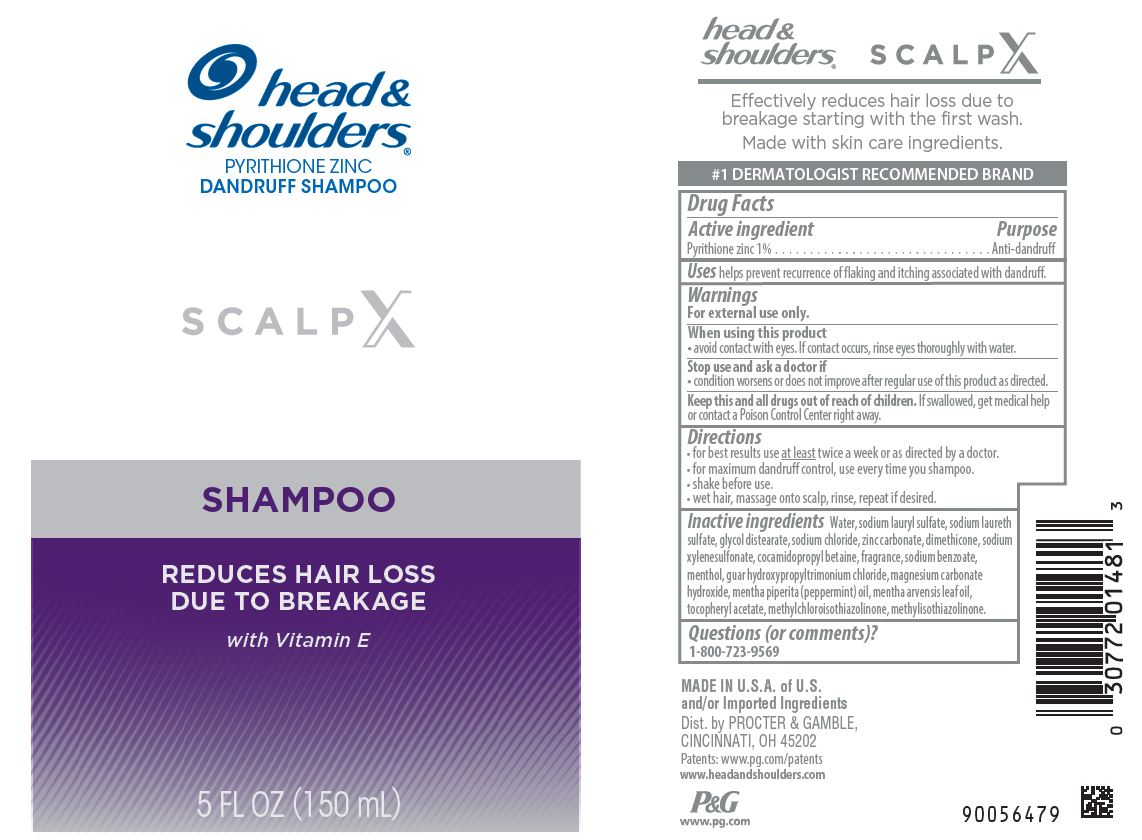
Head and Shoulders ® Scalp X Shampoo
Head and Shoulders Scalp X by
Drug Labeling and Warnings
Head and Shoulders Scalp X by is a Otc medication manufactured, distributed, or labeled by The Procter & Gamble Manufacturing Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEAD AND SHOULDERS SCALP X- pyrithione zinc shampoo
The Procter & Gamble Manufacturing Company
----------
Head and Shoulders ® Scalp X Shampoo
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- for best results use at least twice a week or as directed by a doctor.
- for maximum dandruff control, use every time you shampoo.
- shake before use.
- wet hair, massage onto scalp, rinse, repeat if desired.
Inactive Ingredients
Water, sodium lauryl sulfate, sodium laureth sulfate, glycol distearate, sodium chloride, zinc carbonate, dimethicone, sodium xylenesulfonate, cocamidopropyl betaine, fragrance, sodium benzoate, menthol, guar hydroxypropyltrimonium chloride, magnesium carbonate hydroxide, mentha piperita (peppermint) oil, mentha arvensis leaf oil, tocopheryl acetate, methylchloroisothiazolinone, methylisothiazolinone
| HEAD AND SHOULDERS SCALP X
pyrithione zinc shampoo |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Procter & Gamble Manufacturing Company | 081329183 | manufacture(69423-643) , pack(69423-643) | |
Revised: 12/2023
Document Id: 0c92b8c4-4cff-5478-e063-6294a90a7c3e
Set id: ecf77aae-8216-f2bb-e053-2995a90a74f2
Version: 2
Effective Time: 20231215
The Pr
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.