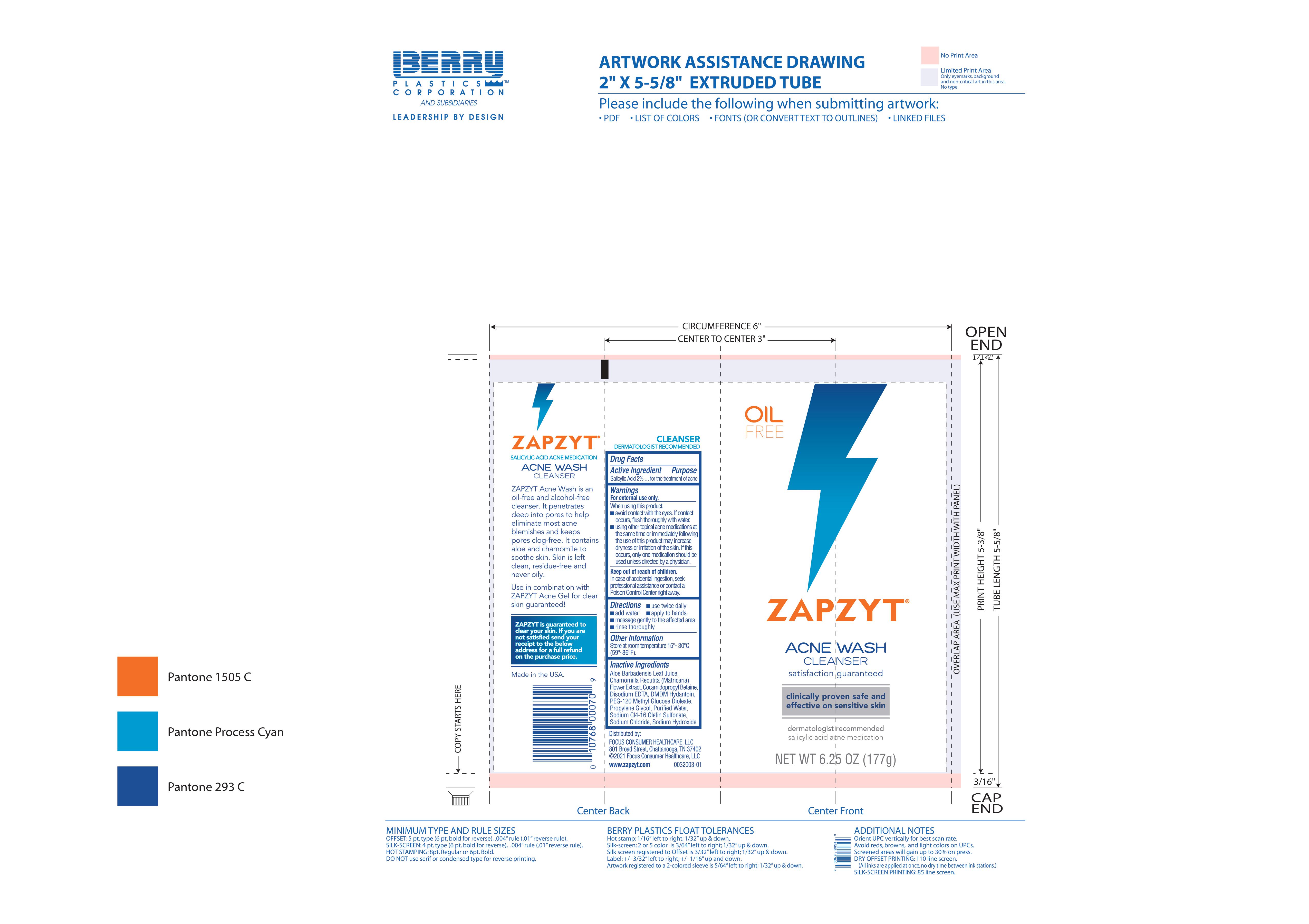
Zapzyt Acne Wash Cleanser
Zapzyt Acne Wash Cleanser by
Drug Labeling and Warnings
Zapzyt Acne Wash Cleanser by is a Otc medication manufactured, distributed, or labeled by Denison Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZAPZYT ACNE WASH CLEANSER- salicylic acid gel
Denison Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Zapzyt Acne Wash Cleanser
Warnings
For external use only.
Avoid contact with eyes. If contact occurs, flush thoroughly with water.
using other topical acne medications at the same time or immediately following the use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a physician.
Directions
- use twice daily
- apply to hands
- add water
- massage gently to the affected area
- rinse thoroughly
| ZAPZYT ACNE WASH CLEANSER
salicylic acid gel |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Denison Pharmaceuticals, LLC (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, LLC | 001207208 | manufacture(0295-9055) | |