POTASSIUM CHLORIDE- potassium chloride solution
POTASSIUM CHLORIDE by
Drug Labeling and Warnings
POTASSIUM CHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Safecor Health, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POTASSIUM CHLORIDE ORAL SOLUTION safely and effectively. See full prescribing information for POTASSIUM CHLORIDE ORAL SOLUTION.
POTASSIUM CHLORIDE oral solution
Initial U.S. Approval: 1948INDICATIONS AND USAGE
Potassium chloride oral solution is indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis, in patients for whom dietary management with potassium-rich foods or diuretic dose reduction are insufficient. (1)
DOSAGE AND ADMINISTRATION
Dilute prior to administration. (2.1, 5.1)
Monitor serum potassium and adjust dosage accordingly. (2.2, 2.3)
Treatment of hypokalemia:
- Adults: Initial doses range from 40 to 100 mEq/day in 2 to 5 divided doses: limit doses to 40 mEq per dose. Total daily dose should not exceed 200 mEq. (2.2)
- Pediatric patients aged birth to 16 years old: 2 to 4 mEq/kg/day in divided doses; not to exceed 1 mEq/kg as a single dose or 20 mEq whichever is lower; if deficits are severe or ongoing losses are great, consider intravenous therapy. Total daily dose should not exceed 100 mEq. (2.3)
Maintenance or Prophylaxis of hypokalemia:
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Concomitant use with potassium sparing diuretics. (4)
WARNINGS AND PRECAUTIONS
- Gastrointestinal Irritation: Dilute before use, take with meals. (5.1)
ADVERSE REACTIONS
Most common adverse reactions are nausea, vomiting, flatulence, abdominal pain/discomfort, and diarrhea. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Apotex Corp. at 1-800-706-5575 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration and Monitoring
2.2 Adult Dosing
2.3 Pediatric Dosing
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Irritation
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Potassium-Sparing Diuretics
7.2 Angiotensin-Converting Enzyme Inhibitors
7.3 Angiotensin Receptor Blockers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Nursing Mothers
8.3 Pediatric Use
8.4 Geriatric Use
10 OVERDOSAGE
10.1 Symptoms
10.2 Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration and Monitoring
Monitoring
Monitor serum potassium and adjust dosages accordingly. For treatment of hypokalemia, monitor potassium levels daily or more often depending on the severity of hypokalemia until they return to normal. Monitor potassium levels monthly-to-biannually for maintenance or prophylaxis.
The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease, or acidosis requires careful attention to acid-base balance, volume status, electrolytes, including magnesium, sodium, chloride, phosphate, and calcium, electrocardiograms and the clinical status of the patient. Correct volume status, acid-base balance and electrolyte deficits as appropriate.
Administration
Dilute the potassium chloride solution with at least 4 ounces of cold water [see Warnings and Precautions (5.1)].
Take with meals or immediately after eating.
If serum potassium concentration is less than 2.5 mEq/L, use intravenous potassium instead of oral supplementation.
2.2 Adult Dosing
Treatment of hypokalemia
Daily dose range from 40 to 100 mEq. Give in 2 to 5 divided doses: limit doses to 40 mEq per dose. The total daily dose should not exceed 200 mEq in a 24 hour period.
Maintenance or Prophylaxis
Typical dose is 20 mEq per day. Individualize dose based upon serum potassium levels.
Studies support the use of potassium replacement in digitalis toxicity. When alkalosis is present, normokalemia and hyperkalemia may obscure a total potassium deficit. The advisability of use of potassium replacement in the setting of hyperkalemia is uncertain.
2.3 Pediatric Dosing
Treatment of hypokalemia
Pediatric patients aged birth to 16 years old: The initial dose is 2 to 4 mEq/kg/day in divided doses; do not exceed as a single dose 1 mEq/kg or 40 mEq, whichever is lower; maximum daily doses should not exceed 100 mEq. If deficits are severe or ongoing losses are great, consider intravenous therapy.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Irritation
May cause gastrointestinal irritation if administered undiluted. Increased dilution of the solution and taking with meals may reduce gastrointestinal irritation [see Dosage and Administration (2.1)].
- 6 ADVERSE REACTIONS
-
7 DRUG INTERACTIONS
7.1 Potassium-Sparing Diuretics
Use with potassium-sparing diuretic can produce severe hyperkalemia. Avoid concomitant use.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with potassium chloride. It is unlikely that potassium supplementation that does not lead to hyperkalemia would have an adverse effect on the fetus or would affect reproductive capacity.
8.2 Nursing Mothers
The normal potassium ion content of human milk is about 13 mEq per liter. Since oral potassium becomes part of the body potassium pool, so long as body potassium is not excessive, the contribution of potassium chloride supplementation should have little or no effect on the level in human milk.
8.3 Pediatric Use
The safety and effectiveness of potassium chloride have been demonstrated in children with diarrhea and malnutrition from birth to18 years.
8.4 Geriatric Use
Clinical studies of potassium chloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
10 OVERDOSAGE
10.1 Symptoms
The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are impaired or if potassium is administered too rapidly potentially fatal hyperkalemia can result.
Hyperkalemia is usually asymptomatic and may be manifested only by an increased serum potassium concentration (6.5 to 8.0 mEq/L) and characteristic electrocardiographic changes (peaking of T-waves, loss of P-waves, depression of S-T segment, and prolongation of the QT-interval). Late manifestations include muscle paralysis and cardiovascular collapse from cardiac arrest (9 to12 mEq/L).
10.2 Treatment
Treatment measures for hyperkalemia include the following:
- Monitor closely for arrhythmias and electrolyte changes.
- Eliminate foods and medications containing potassium and of any agents with potassium-sparing properties such as potassium-sparing diuretics, ARBS, ACE inhibitors, NSAIDS, certain nutritional supplements and many others.
- Administer intravenous calcium gluconate if the patient is at no risk or low risk of developing digitalis toxicity.
- Administer intravenously 300 to 500 mL/hr of 10% dextrose solution containing 10 to 20 units of crystalline insulin per 1000 mL.
- Correct acidosis, if present, with intravenous sodium bicarbonate.
- Use exchange resins, hemodialysis, or peritoneal dialysis.
In patients who have been stabilized on digitalis, too rapid a lowering of the serum potassium concentration can produce digitalis toxicity.
-
11 DESCRIPTION
Potassium chloride, USP is a colorless, elongated, prismatic, or cubical crystals, or white, granular powder. It is soluble in water and slightly soluble in alcohol. Chemically, potassium chloride is K-Cl with a molecular mass of 74.55 g/mol.
Oral Solution 10%: Each 15 mL of solution contains 1.5 g of potassium chloride, USP and the following inactive ingredients: citric acid anhydrous, FD&C Yellow #6, glycerin, methylparaben, natural orange flavor, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, sucralose.
Oral Solution 20%: Each 15 mL of solution contains 3.0 g of potassium chloride, USP and the following inactive ingredients: citric acid anhydrous, FD&C Yellow #6, glycerin, methylparaben, natural orange flavor, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, sucralose.
Natural orange flavor includes fruit extract and natural flavor.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The potassium ion (K+) is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes including the maintenance of intracellular tonicity; the transmission of nerve impulses; the contraction of cardiac, skeletal, and smooth muscle; and the maintenance of normal renal function.
The intracellular concentration of potassium is approximately 150 to 160 mEq per liter. The normal adult plasma concentration is 3.5 to 5 mEq per liter. An active ion transport system maintains this gradient across the plasma membrane.
Potassium is a normal dietary constituent, and under steady-state conditions the amount of potassium absorbed from the gastrointestinal tract is equal to the amount excreted in the urine. The usual dietary intake of potassium is 50 to 100 mEq per day.
12.3 Pharmacokinetics
Based on published literature, the rate of absorption and urinary excretion of potassium from KCl oral solution were higher during the first few hours after dosing relative to modified release KCl products. The bioavailability of potassium, as measured by the cumulative urinary excretion of K+ over a 24 hour post dose period, is similar for KCl solution and modified release products.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Potassium chloride oral solution, USP is an orange solution available in two strengths as follows:
10%: 20 mEq/15 mL oral solution
NDC# 48433-217-15 Unit Dose Cup of 15 mL
NDC# 48433-217-40 Box of 40 Unit Dose Cups, each with 15 mL20%: 40 mEq/15 mL oral solution
NDC# 48433-218-15 Unit Dose Cup of 15 mL
NDC# 48433-218-40 Box of 40 Unit Dose Cups, each with 15 mLFor Institutional Use Only
This Package is Not Child ResistantStorage
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
PROTECT from LIGHT.
POTASSIUM CHLORIDE ORAL SOLUTION, USP, 10% and 20%
Packaged by:
Safecor Health, LLC
4060 Business Park Drive
Columbus, OH 43204Manufactured by:
Apotex Inc.
Toronto, Ontario
Canada M9L 1T9Manufactured for:
Apotex Corp.
Weston, Florida
USA 33326Rev: 03/2019 PN5644
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 10% Box Label
SAFECOR
HEALTHNDC: 48433-217-40
Potassium Chloride Oral Solution USP 10%
Contains 40 (15mL) Unit Dose Cups
See package insert for complete drug information
Store at 20° to 25°C (68° to 77°F) See USP Controlled Room Temperature
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDRENRx ONLY
FOR INSTITUTIONAL USE ONLY
Pkg By: Safecor Health, LLC Columbus, OH 43204.
Questions or Comments: Cal 1-800-447-1006
GTN: 00348433217405
SN: 192000028
Exp: 201212
Lot: SC0508195682.A
-
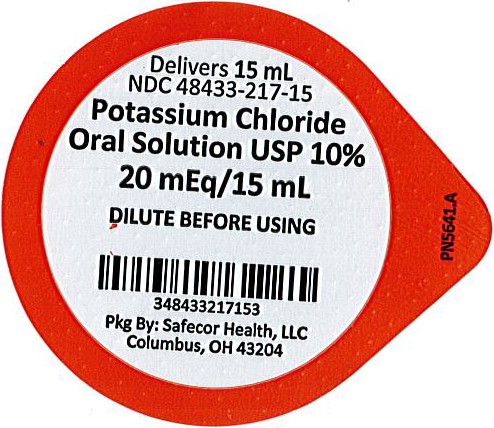
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 10% Lid Label
Delivers 15mL
NDC: 48433-217-15
Potassium Chloride Oral Solution USP 10%
20 mEq/15 mL
DILUTE BEFORE USING
Pkg By: Safecor Health, LLC
Columbus, OH 43204PN5641.A
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 10% Box Label
SAFECOR
HEALTHNDC: 48433-218-40
Potassium Chloride Oral Solution USP 20%
Contains 40 (15mL) Unit Dose Cups
See package insert for complete drug information
Store at 20° to 25°C (68° to 77°F) See USP Controlled Room Temperature
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDRENRx ONLY
FOR INSTITUTIONAL USE ONLY
Pkg By: Safecor Health, LLC Columbus, OH 43204.
Questions or Comments: Cal 1-800-447-1006
GTN: 00348433218402
SN: 192000031
Exp: 201213
Lot: CS0508195682.A
-
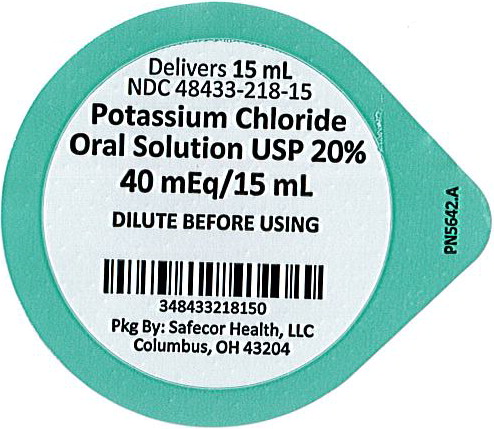
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 20% Lid Label
Delivers 15mL
NDC: 48433-218-15
Potassium Chloride Oral Solution USP 20%
40 mEq/15 mL
DILUTE BEFORE USING
Pkg By: Safecor Health, LLC
Columbus, OH 43204PN5641.A
-
INGREDIENTS AND APPEARANCE
POTASSIUM CHLORIDE
potassium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 48433-217(NDC:60505-6184) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152, Chloride Ion - UNII:Q32ZN48698) Potassium Chloride 20 meq in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Product Characteristics Color ORANGE Score Shape Size Flavor ORANGE (ORANGE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48433-217-40 40 in 1 BOX, UNIT-DOSE 05/24/2019 1 NDC: 48433-217-15 15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211067 05/24/2019 POTASSIUM CHLORIDE
potassium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 48433-218(NDC:60505-6185) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152, Chloride Ion - UNII:Q32ZN48698) Potassium Chloride 40 meq in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Product Characteristics Color ORANGE Score Shape Size Flavor ORANGE (ORANGE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48433-218-40 40 in 1 BOX, UNIT-DOSE 05/24/2019 1 NDC: 48433-218-15 15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211067 05/24/2019 Labeler - Safecor Health, LLC (828269675) Establishment Name Address ID/FEI Business Operations Safecor Health, LLC 828269675 repack(48433-217, 48433-218)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.