Premier Value 44-104-Delisted
Pain Reliever by
Drug Labeling and Warnings
Pain Reliever by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAIN RELIEVER- acetaminophen tablet
Chain Drug Consortium
----------
Premier Value 44-104-Delisted
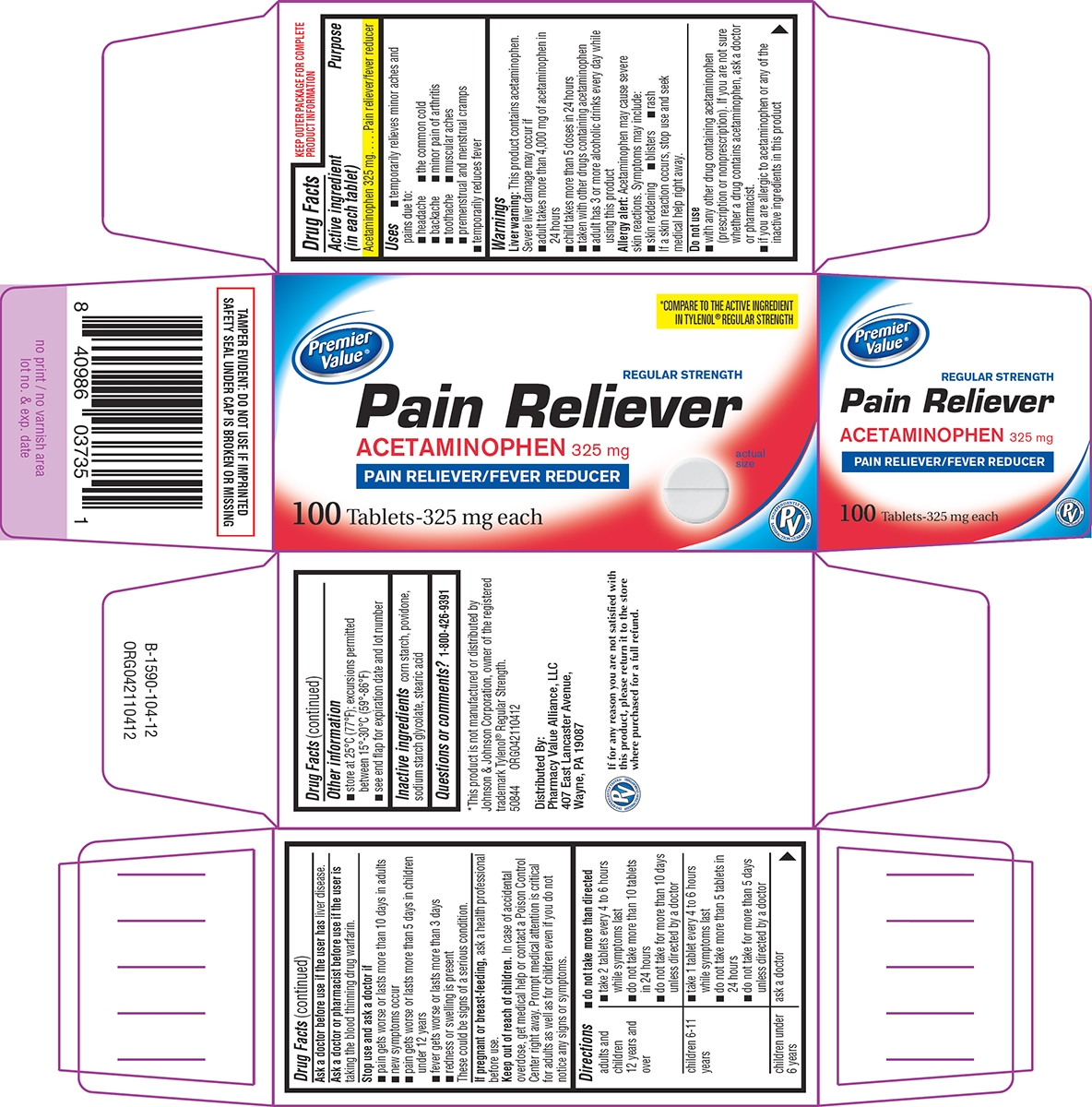
Uses
- temporarily relieves minor aches and pains due to:
- headache
- the common cold
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- do not take more than directed
| adults and children 12 years and over |
■ take 2 tablets every 4 to 6 hours while symptoms last |
| children 6-11 years |
■ take 1 tablet every 4 to 6 hours while symptoms last |
| children under 6 years | ask a doctor |
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Principal display panel
Premier
Value®
*COMPARE TO THE ACTIVE INGREDIENT
IN TYLENOL® REGULAR STRENGTH
REGULAR STRENGTH
Pain Reliever
ACETAMINOPHEN 325 mg
PAIN RELIEVER/FEVER REDUCER
actual
size
100 Tablets-325 mg each
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the registered
trademark Tylenol® Regular Strength.
50844 ORG042110412
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
If for any reason you are not satisfied with
this product, please return it to the store
where purchased for a full refund.
Premier Value 44-104
| PAIN RELIEVER
acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(68016-541) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(68016-541) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(68016-541) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.