BARE REPUBLIC MINERAL MATTE TINTED FACE SPF 30- titanium dioxide, zinc oxide lotion
Bare Republic Mineral Matte Tinted Face SPF 30 by
Drug Labeling and Warnings
Bare Republic Mineral Matte Tinted Face SPF 30 by is a Otc medication manufactured, distributed, or labeled by COOLA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
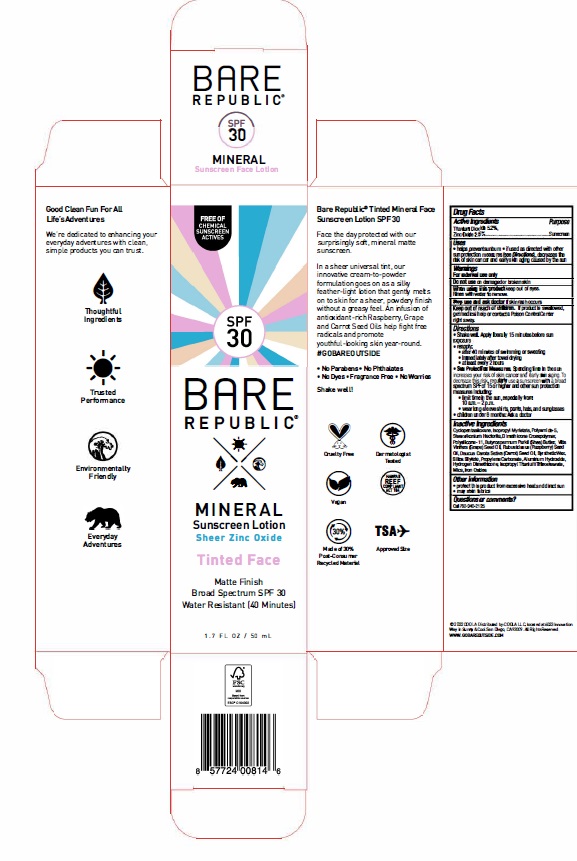
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Shake well. Apply liberally 15 minutes before sun exposure.
- Reapply: after 40 minutes of swimming or sweating immediately afrer towel drying at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from: 10 a.m. -2 p.m. wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Cyclopentasiloxane, Isopropyl Myristate, Polyamide-5, Stearalkonium Hectorite, Dimethicone Crosspolymer, Polysilicone-11, Butyrospermum Parkii (Shea) Butter, Vitis Vinifera (Grape) Seed Oil, Rubus Idaeus (Raspberry) Seed Oil, Daucus Carota Sativa (Carrot) Seed Oil, Synthetic Wax, Silica Silylate, Propylene Carbonate, Aluminum Hydroxide, Hydrogen Dimethicone, Isopropyl Titanium Triisostearate, Mica, Iron Oxides
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BARE REPUBLIC MINERAL MATTE TINTED FACE SPF 30
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 79753-065 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5.2 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.5 g in 100 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SHEA BUTTER (UNII: K49155WL9Y) CARROT SEED OIL (UNII: 595AO13F11) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYAMIDE-5 (UNII: UEM62QMW5F) RASPBERRY SEED OIL (UNII: 9S8867952A) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) GRAPE SEED OIL (UNII: 930MLC8XGG) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) SYNTHETIC WAX (1800 MW) (UNII: 248P1AUJ90) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 79753-065-01 1 in 1 CARTON 01/01/2019 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2019 Labeler - COOLA, LLC (956990290)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.