NAFTIFINE HYDROCHLORIDE cream
Naftifine Hydrochloride by
Drug Labeling and Warnings
Naftifine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Renaissance Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
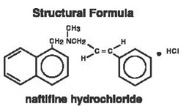
DESCRIPTION
Naftifine Hydrochloride Cream, 1% contains the synthetic, broad-spectrum, antifungal agent naftifine hydrochloride. Naftifine Hydrochloride Cream, 1% is for topical use only.
-
CLINICAL PHARMACOLOGY
Naftifine hydrochloride is a synthetic allylamine derivative. The following in vitro data are available, but their clinical significance is unknown. Naftifine hydrochloride has been shown to exhibit fungicidal activity in vitro against a broad spectrum of organisms, including Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Epidermophyton floccosum, Microsporum canis, Microsporum audouini, and Microsporum gypseum; and fungistatic activity against Candida species, including Candida albicans. Naftifine Hydrochloride Cream, 1% has only been shown to be clinically effective against the disease entities listed in the INDICATIONS AND USAGE section.
Although the exact mechanism of action against fungi is not known, naftifine hydrochloride appears to interfere with sterol biosynthesis by inhibiting the enzyme squalene 2, 3-epoxidase. This inhibition of enzyme activity results in decreased amounts of sterols, especially ergosterol, and a corresponding accumulation of squalene in the cells.
Pharmacokinetics
In vitro and in vivo bioavailability studies have demonstrated that naftifine penetrates the stratum corneum in sufficient concentration to inhibit the growth of dermatophytes.
Following a single topical application of 1% naftifine cream to the skin of healthy subjects, systemic absorption of naftifine was approximately 6% of the applied dose. Naftifine and/or its metabolites are excreted via the urine and feces with a half-life of approximately two to three days.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
Naftifine Hydrochloride Cream, 1% is for external use only. If irritation or sensitivity develops with the use of Naftifine Hydrochloride Cream, 1%, treatment should be discontinued and appropriate therapy instituted. Diagnosis of the disease should be confirmed either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide or by culture on an appropriate medium.
Information for patients
The patient should be told to:
- 1. Avoid the use of occlusive dressings or wrappings unless otherwise directed by the physician.
- 2. _Keep Naftifine Hydrochloride Cream, 1% away from the eyes, nose, mouth and other mucous membranes.
Carcinogenesis, mutagenesis, impairment of fertility
Long-term animal studies to evaluate the carcinogenic potential of Naftifine Hydrochloride Cream, 1% have not been performed. In vitro and animal studies have not demonstrated any mutagenic effect or effect on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats and rabbits (via oral administration) at doses 150 times or more the topical human dose and have revealed no evidence of impaired fertility or harm to the fetus due to naftifine. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
A sufficient quantity of Naftifine Hydrochloride Cream, 1% should be gently massaged into the affected and surrounding skin areas once a day. The hands should be washed after application. If no clinical improvement is seen after four weeks of treatment with Naftifine Hydrochloride Cream, 1%, the patient should be re-evaluated.
-
HOW SUPPLIED
Naftifine Hydrochloride 1% Cream is supplied in the following sizes:
- 30g—NDC: 40085-201-30 (tube)
- 60g—NDC: 40085-201-60 (tube)
- 90g—NDC: 40085-201-90 (tube)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 g Tube Label
-
INGREDIENTS AND APPEARANCE
NAFTIFINE HYDROCHLORIDE
naftifine hydrochloride creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 40085-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAFTIFINE HYDROCHLORIDE (UNII: 25UR9N9041) (NAFTIFINE - UNII:4FB1TON47A) NAFTIFINE HYDROCHLORIDE 1 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Isopropyl Myristate (UNII: 0RE8K4LNJS) Polysorbate 60 (UNII: CAL22UVI4M) Stearyl Alcohol (UNII: 2KR89I4H1Y) Cetyl Alcohol (UNII: 936JST6JCN) Cetyl Esters Wax (UNII: D072FFP9GU) Sorbitan Monostearate (UNII: NVZ4I0H58X) Benzyl Alcohol (UNII: LKG8494WBH) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 40085-201-60 1 in 1 CARTON 10/01/2016 04/30/2020 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 40085-201-90 1 in 1 CARTON 10/01/2016 04/30/2020 2 90 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019599 10/01/2016 04/30/2020 Labeler - Renaissance Pharma, Inc. (078290398)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.