CRYSELLE- norgestrel and ethinyl estradiol kit
Cryselle by
Drug Labeling and Warnings
Cryselle by is a Prescription medication manufactured, distributed, or labeled by Teva Pharmaceuticals USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Cryselle® (norgestrel and ethinyl estradiol tablets USP)
-
BOXED WARNING
(What is this?)
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age and smoke [see Contraindications].
-
DESCRIPTION
Cryselle® is a combination oral contraceptive containing the progestational compound norgestrel, USP and the estrogenic compound ethinyl estradiol, USP. Norgestrel is designated as (2) (±)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one and ethinyl estradiol is designated as (19-nor-17α-pregna-1,3,5 (10)-trien-20-yne-3,17-diol). Each white active Cryselle tablet contains 0.3 mg norgestrel, USP and 0.03 mg ethinyl estradiol, USP. The inactive ingredients present are hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol and pregelatinized corn starch. The light-green inactive tablets also contain D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake and FD&C Yellow No. 6 Aluminum Lake.
Norgestrel, USP
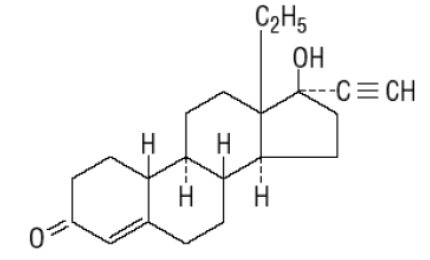
C21H28O2 MW: 312.45
Ethinyl Estradiol, USP
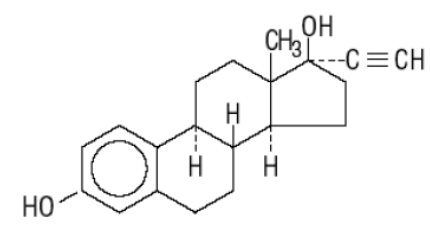
C20H24O2 MW: 296.40
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Do not prescribe Cryselle to women who are known to have any of the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- Smoke, if over age 35
- Have deep-vein thrombosis or pulmonary embolism, now or in the past
- Have inherited or acquired coagulopathies
- Have cerebrovascular disease
- Have coronary artery disease
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease or atrial fibrillation)
- Have uncontrolled hypertension
- Have diabetes mellitus with vascular disease
- Headaches with focal neurological symptoms or migraine headaches with aura
- Women over age 35 with any migraine headaches
- Liver tumors, benign or malignant, or liver disease
- Undiagnosed abnormal uterine bleeding
- Pregnancy, because there is no reason to use COCs during pregnancy
- Breast cancer or other estrogen- or progestin-sensitive cancer, now or in the past
- Hypersensitivity to any of the components of Cryselle
Combination oral contraceptives should not be used in women who are receiving Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations (see Warnings, Risk of liver enzyme elevations with concomitant hepatitis c treatment).
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
-
WARNINGS
1. Thromboembolic Disorders and Other Vascular Problems
- Stop Cryselle if an arterial thrombotic event or venous thromboembolic (VTE) event occurs.
- Stop Cryselle if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
- If feasible, stop Cryselle at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of VTE as well as during and following prolonged immobilization.
- Start Cryselle no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum VTE decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
- The use of COCs increases the risk of VTE. However, pregnancy increases the risk of VTE as much or more than the use of COCs. The risk of VTE in women using COCs is 3 to 9 cases per 10,000 woman-years. The risk of VTE is highest during the first year of use of COCs and when restarting hormonal contraception after a break of 4 weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after use is discontinued.
- Use of COCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. COCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). This risk increases with age, particularly in women over 35 years of age who smoke.
- Use COCs with caution in women with cardiovascular disease risk factors.
2. Liver Disease
Impaired Liver Function
Do not use Cryselle in women with liver disease, such as acute viral hepatitis or severe (decompensated) cirrhosis of the liver [see Contraindications]. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded. Discontinue Cryselle if jaundice develops.
Liver Tumors
Cryselle is contraindicated in women with benign and malignant liver tumors [see Contraindications]. Hepatic adenomas are associated with COC use. An estimate of the attributable risk is 3.3 cases/100,000 users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) COC users. However the risk of liver cancers in COC users approaches less than one case per million users.
Risk Of Liver Enzyme Elevations With Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications such as COCs. Discontinue Cryselle prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications]. Cryselle can be restarted approximately 2 weeks following completion of treatment with the combination drug regimen.
3. High Blood Pressure
Cryselle is contraindicated in women with uncontrolled hypertension or hypertension with vascular disease [see Contraindications]. For women with well-controlled hypertension, monitor blood pressure and stop Cryselle if blood pressure rises significantly.
An increase in blood pressure has been reported in women taking COCs, and this increase is more likely in older women with extended duration of use. The incidence of hypertension increases with increasing quantities of progestin.
4. Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among COC users. Use of COCs may worsen existing gallbladder disease. A past history of COC-related cholestasis predicts an increased risk with subsequent COC use. Women with a history of pregnancy-related cholestasis may be at an increased risk for COC related cholestasis.
5. Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who take Cryselle. COCs may decrease glucose tolerance.
Consider alternative contraception for women with uncontrolled dyslipidemia. A small proportion of women will have adverse lipid changes while on COCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
6. Headache
If a woman taking Cryselle develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue Cryselle if indicated.
Consider discontinuation of Cryselle in the case of increased frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event).
7. Bleeding Irregularities and Amenorrhea
Unscheduled Bleeding and Spotting
Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on COCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different contraceptive product.
In 1,287 patients (pooled data from a number of studies), unscheduled bleeding was recorded in 15% of first cycles and by Cycle 12 was 5%. In total, 23% of subjects reported spotting, 20% reported unscheduled bleeding, and 2% reported change in menstrual flow at some point in the studies.
In the studies, 1.2% discontinued use of the product due to breakthrough bleeding and 1% discontinued due to spotting.
Amenorrhea and Oligomenorrhea
Women who use Cryselle may experience amenorrhea. A total of 9% of subjects in the studies reported amenorrhea in one or more cycles.
Some women may experience amenorrhea or oligomenorrhea after discontinuation of COCs, especially when such a condition was preexistent. If scheduled (withdrawal) bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
-
PRECAUTIONS
1. Carcinoma of the Breast and Cervix
- Cryselle is contraindicated in women who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications].
- There is substantial evidence that COCs do not increase the incidence of breast cancer. Although some studies suggest that COCs might increase the incidence of breast cancer, more recent studies have not confirmed such findings.
- Some studies suggest that COC use has been associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
2. Effect on Binding Globulins
The estrogen component of COCs may raise the serum concentrations of thyroxine-binding globulin, sex hormone-binding globulin, and cortisol-binding globulin. The dose of replacement thyroid hormone or cortisol therapy may need to be increased.
3. Hereditary Angioedema
In women with hereditary angioedema, exogenous estrogens may induce or exacerbate symptoms of angioedema.
4. Chloasma
Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation while taking Cryselle.
5. Drug Interactions
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation:
Do not coadminister Cryselle with HCV drug combinations containing ombitasvir/ paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations (see Warnings, Risk of liver enzyme elevations with concomitant hepatitis c treatment).
Effects of Other Drugs on Combined Oral Contraceptives
Substances decreasing the plasma concentrations of COCs and potentially diminishing the efficacy of COCs: Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the plasma concentrations of COCs and potentially diminish the effectiveness of COCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate rifabutin, rufinamide, aprepitant, and products containing St. John’s wort. Interactions between hormonal contraceptives and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative method of contraception or a back-up method when enzyme inducers are used with COCs, and to continue back-up contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Colesevelam: Colesevelam, a bile acid sequestrant, given together with a COC, has been shown to significantly decrease the AUC of EE. The drug interaction between the contraceptive and colesevelam was decreased when the two drug products were given 4 hours apart.
Substances increasing the plasma concentrations of COCs: Co-administration of atorvastatin or rosuvastatin and certain COCs containing EE increase AUC values for EE by approximately 20 to 25%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation. Concomitant administration of CYP3A4 inhibitors such as itraconazole, fluconazole, grapefruit juice or ketoconazole may increase plasma hormone concentrations.
Human immunodeficiency virus (HIV)/ Hepatitis C virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma concentrations of the estrogen and/or progestin have been noted when COCs are coadministered with some HIV protease inhibitors (decrease [e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir], or increase [e.g., indinavir and atazanavir/ritonavir] HCV protease inhibitors (decrease [e.g., nevirapine] or increase [e.g., etravirine]).
Effects of Combined Oral Contraceptives on Other Drugs
COCs containing EE may inhibit the metabolism of other drugs (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole) and increase their plasma concentrations. COCs have been shown to decrease plasma concentrations of acetaminophen, clofibric acid, morphine, salicylic acid, temazepam and lamotrigine. Significant decrease in the plasma concentration of lamotrigine has been shown, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary.
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentration of thyroid-binding globulin increases with use of COCs.
6. Interference with Laboratory Tests
The use of contraceptive steroids may influence the results of certain laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins.
8. Pregnancy
There is little or no increased risk of birth defects in women who inadvertently use COCs during early pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb reduction defects) following exposure to low dose COCs prior to conception or during early pregnancy.
Discontinue Cryselle use if pregnancy is confirmed.
Do not administer COCs to induce withdrawal bleeding as a test for pregnancy. Do not use COCs during pregnancy to treat threatened or habitual abortion.
9. Nursing Mothers
Advise the nursing mother to use other forms of contraception, when possible, until she has weaned her child. COCs can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women. Small amounts of oral contraceptive steroids and/or metabolites are present in breast milk.
10. Pediatric Use
Safety and efficacy of Cryselle tablets have been established in women of reproductive age. Efficacy is expected to be the same for post-pubertal adolescents under the age of 16 and for users 16 years and older. Use of Cryselle before menarche is not indicated.
11. Geriatric Use
Cryselle has not been studied in postmenopausal women and is not indicated in this population.
12. Information for the Patient
See FDA-approved patient labeling (Patient Information and Instructions for Use). Counsel patients about the following information:
- Cigarette smoking increases the risk of serious cardiovascular events from COC use, and that women who are over 35 years old and smoke should not use COCs [see Boxed Warning].
- Increased risk of VTE compared to non-users of COCs is greatest after initially starting a COC or restarting (following a 4-week or greater pill-free interval) the same or a different COC.
- Cryselle does not protect against HIV infection and other sexually transmitted infections.
- Cryselle is not to be used during pregnancy; if pregnancy occurs during use of Cryselle, instruct the patient to stop further use.
- Take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event tablets are missed.
- Use a back-up or alternative method of contraception when enzyme inducers are used with Cryselle.
- COCs may reduce breast milk production; this is less likely to occur if breastfeeding is well established.
- Women who start COCs postpartum, and who have not yet had a period, should use an additional method of contraception until they have taken an active tablet for 7 consecutive days.
- Amenorrhea may occur. Consider pregnancy in the event of amenorrhea at the time of the first missed period. Rule out pregnancy in the event of amenorrhea in two or more consecutive cycles.
-
ADVERSE REACTIONS
An increased risk of the following serious adverse reactions (see Warnings section for additional information) has been associated with the use of oral contraceptives:
- Serious cardiovascular events and stroke [see Boxed Warning]
- Vascular events
- Liver disease
Adverse reactions commonly reported by COC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of Cryselle was evaluated in 1,343 healthy women of child-bearing potential who participated in 9 clinical trials and received at least one dose of Cryselle for contraception. Subjects were exposed for a total of 11,085 cycles, with 429 women completing one year of exposure. Subjects ranged in age from 15 to 40 years. Demographics were 69% Caucasian, 28% Black, and 3% other.
Common Adverse Reactions (≥ 2% of women):
- Weight increase (11%)
- Cervical erosion (9%)
- Weight decrease (6%)
- Acne (4%)
- Dysmenorrhea (4%)
- Vaginal discharge (4%)
- Abdominal pain, cramps, and bloating (3%)
- Appetite increase (3%)
- Depression (3%)
- Nervousness (3%)
- Chloasma/melasma (2%)
- Fatigue (2%)
- Varicose veins, aggravation of (2%)
A total of 8% of subjects discontinued the trials prematurely due to an adverse reaction, most commonly due to unscheduled bleeding, spotting, headache (including migraine), nausea, acne, changes in menstrual flow, weight increase, nervousness, high blood pressure, and depression.
Postmarketing Experience
The following additional adverse drug reactions have been reported from worldwide postmarketing experience with Cryselle. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Arterial Events: Arterial thromboembolism, Myocardial infarction, Cerebral hemorrhage
Eye Disorder: Optic neuritis, which may lead to partial or complete loss of vision, Intolerance to contact lenses, Change (steepening) in corneal curvature
Gastrointestinal Disorders: Colitis, Nausea, Pancreatitis
Hepatobiliary Disorders: Gallbladder disease, Cholestatic jaundice, Budd-Chiari syndrome
Immune System Disorders: Anaphylactic/anaphylactoid reactions, including urticaria, angioedema, and severe reactions with respiratory and circulatory symptoms
Metabolism and Nutrition Disorders: Carbohydrate and lipid effects, Porphyria, exacerbation of Porphyria
Neoplasms, Benign, Malignant, and Unspecified: Carcinoma of the reproductive organs and breasts, Hepatic neoplasia (including hepatic adenomas or benign liver tumors)
Psychiatric Disorders: Mood changes
Reproductive System and Breast Disorders: Temporary infertility after discontinuation of treatment, Changes in libido, Vaginitis, including candidiasis; Breast secretion
Skin and Subcutaneous Tissue Disorders: Melasma/chloasma, which may persist; Erythema multiforme, Erythema nodosum, Hemorrhagic eruption, Hirsutism Vascular Events: Venous thrombosis, Pulmonary embolism, Cerebral thrombosis, Mesenteric thrombosis, Retinal vascular thrombosis
OVERDOSAGE
There have been no reports of serious ill effects from overdosage of oral contraceptives, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea.
DOSAGE AND ADMINISTRATION
To achieve maximum contraceptive effectiveness, Cryselle (norgestrel and ethinyl estradiol tablets) must be taken exactly as directed and at intervals not exceeding 24 hours. The dosage of Cryselle is one white tablet daily for 21 consecutive days, followed by one light-green colored inert tablet daily for 7 consecutive days, according to prescribed schedule. It is recommended that Cryselle tablets be taken by mouth at the same time each day.
How to Start Cryselle
Consider the possibility of ovulation and conception prior to initiation of medication.
Instruct the patient to begin taking Cryselle on the first Sunday after the onset of menstruation. If menstruation begins on a Sunday, the first tablet (white) is taken that day. The patient should take one white tablet daily for 21 consecutive days followed by one light-green colored inert tablet daily for 7 consecutive days. Withdrawal bleeding will usually occur within 3 days following discontinuation of white tablets and may not have finished before the next pack is started. During the first cycle, the patient should not rely on Cryselle for contraception until a white tablet has been taken daily for 7 consecutive days and she should use a non-hormonal back-up method of birth control during those 7 days.
After the first cycle of use
The patient is to begin her next and all subsequent 28-day courses of tablets on the same day of the week (Sunday) on which she began her first course, following the same schedule: 21 days of white tablets, followed by 7 days of light-green colored inert tablets. If in any cycle the patient starts tablets later than the proper day, instruct her to protect herself against pregnancy by using a non-hormonal back-up method of birth control until she has taken a white tablet daily for 7 consecutive days.
Switching from another hormonal method of contraception
- When the patient is switching from a 21-day regimen of tablets, instruct her to wait 7 days after her last tablet before she starts Cryselle. She will probably experience withdrawal bleeding during that week. Instruct her not to let more than 7 days pass after her previous 21-day regimen before she starts Cryselle.
- When the patient is switching from a 28-day regimen of tablets, instruct her to start her first pack of Cryselle on the day after her last tablet. She should not wait any days between packs.
- The patient may switch any day from a progestin-only pill and should begin Cryselle the next day. If switching from an implant or injection, instruct the patient to start Cryselle on the day of implant removal or the day the next injection would be due. If switching from a contraceptive vaginal ring or transdermal patch instruct the patient to start Cryselle on the day they would have inserted the next ring or applied the next patch. If switching from an Intrauterine Device (IUD) or Intrauterine System (IUS), instruct the patient to start Cryselle on the day of IUD/IUS removal. If the IUD/IUS is not removed on the first day of the patient’s menstrual cycle, instruct her to use a non-hormonal back-up method of birth control for the first 7 days of tablet-taking.
Use after pregnancy, abortion, or miscarriage
- Initiate Cryselle no earlier than day 28 postpartum in the nonlactating mother or after a second-trimester abortion due to the increased risk for thromboembolism (see Contraindications, Warnings and Precautions concerning thromboembolic disease). Advise the patient to use a non-hormonal back-up method for the first 7 days of tablet-taking.
- Cryselle may be initiated immediately after a first-trimester abortion or miscarriage. If the patient starts Cryselle immediately, back-up contraception is not needed.
If spotting or breakthrough bleeding occurs
If spotting or breakthrough bleeding occurs, instruct the patient to continue on the same regimen. This type of bleeding is usually transient and without significance; however, advise the patient to consult her healthcare provider if the bleeding is persistent or prolonged.
Missed Tablets
The possibility of ovulation and pregnancy increases with each successive day that scheduled white tablets are missed. If withdrawal bleeding does not occur, the possibility of pregnancy must be considered. If the patient has not adhered to the prescribed schedule (if she missed one or more tablets or started taking them on a day later than she should have), consider the probability of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
For additional patient instructions regarding missed tablets, see the WHAT TO DO IF YOU MISS PILLS section in FDA-Approved Patient Labeling below.
Advice in Case of Gastrointestinal Disturbances
In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting or diarrhea occurs within 3 to 4 hours after taking an active tablet, handle this as a missed tablet [see FDA-Approved Patient Labeling].
-
HOW SUPPLIED
Cryselle® (norgestrel and ethinyl estradiol tablets USP), 0.3 mg/0.03 mg are available in packages of 6 blister card dispensers (NDC: 0555-9049-58), each containing 28 tablets as follows: 21 active, white, round, film-coated, biconvex tablets debossed with dp on one side and 543 on the other side and 7 inert, round, light-green colored, uncoated tablets debossed dp and 331.
Store at 20º to 25°C (68° to 77º F) [See USP Controlled Room Temperature].
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Rev. C 12/2017
-
Patient Package Insert
FDA-Approved Patient Labeling
Cryselle (norgestrel and ethinyl estradiol tablets)
What is the most important information I should know about Cryselle?
Do not use Cryselle if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects from hormonal birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
What is Cryselle?
Cryselle is a birth control pill (oral contraceptive) used by women to prevent pregnancy.
How does Cryselle work for contraception?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
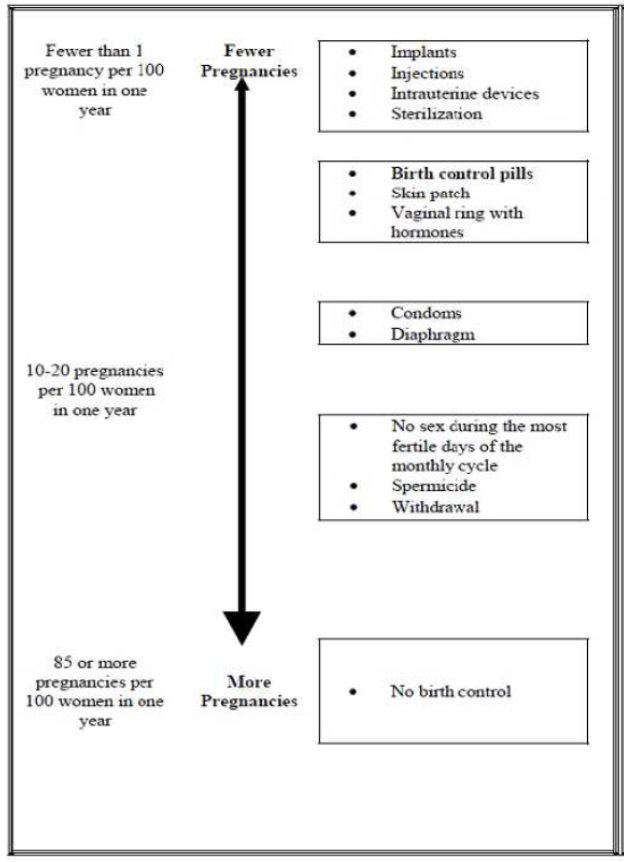
Based on the results of clinical studies, about 1 out of 100 women may get pregnant during the first year they use Cryselle.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
Who should not take Cryselle?
Do not take Cryselle if you:
- smoke and are over 35 years old
- had blood clots in the legs, arms, lungs, or eyes
- had a problem with your blood that makes it clot more than normal
- have certain heart valve problems or irregular heart beat that increases your risk of having blood clots
- had a stroke
- had a heart attack
- have high blood pressure that cannot be controlled by medicine
- have diabetes with kidney, eye, nerve or blood vessel damage
- have certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision, or have any migraine headaches if you are over 35 years of age
- have liver problems, including liver tumors
- have any unexplained vaginal bleeding
- are pregnant
- have or had breast cancer or any cancer that is sensitive to female hormones
- have a known allergy or hypersensitivity to any of the components of Cryselle (norgestrel and ethinyl estradiol tablets)
You should not take the pill if you take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood.
If any of these conditions happen while you are taking Cryselle, stop taking Cryselle right away and talk to your healthcare provider. Use non-hormonal contraception when you stop taking Cryselle.
What should I tell my healthcare provider before taking Cryselle?
Tell your healthcare provider if you:
- are pregnant or think you may be pregnant
- are depressed now or have been depressed in the past
- had yellowing of your skin or eyes (jaundice) caused by pregnancy (cholestasis of pregnancy)
- are breastfeeding or plan to breastfeed. Cryselle may decrease the amount of breast milk you make. A small amount of the hormones in Cryselle may pass into your breast milk. Talk to your healthcare provider about the best birth control method for you while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Cryselle may affect the way other medicines work, and other medicines may affect how well Cryselle works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Cryselle?
Read the Instructions for Use at the end of this Patient Information.
What are the possible serious side effects of Cryselle?
- Like pregnancy, Cryselle may cause serious side effects, including blood clots in your lungs , heart attack, or a stroke that may lead to death.
- Some other examples of serious blood clots include blood clots in the legs or eyes.
Serious blood clots can happen especially if you smoke, are obese, or are older than 35 years of age. Serious blood clots are more likely to happen when you:
- first start taking birth control pills
- restart the same or different birth control pills after not using them for a month or more
Call your healthcare provider or go to a hospital emergency room right away if you have:
- leg pain that will not go away
- sudden severe shortness of breath
- sudden change in vision or blindness
- chest pain
- a sudden, severe headache unlike your usual headaches
- weakness or numbness in your arm or leg
- trouble speaking
Other serious side effects include:
-
liver problems, including:
- rare liver tumors
- jaundice (cholestasis), especially if you previously had cholestasis of pregnancy. Call your healthcare provider if you have yellowing of your skin or eyes.
- high blood pressure
- gallbladder problems
- changes in the sugar and fat (cholesterol and triglycerides ) levels in your blood
- new or worsening headaches including migraine headaches
- depression
- possible cancer in your breast and cervix
- swelling of your skin especially around your mouth, eyes, and in your throat (angioedema). Call your healthcare provider if you have a swollen face, lips, mouth tongue or throat, which may lead to difficulty swallowing or breathing. Your chance of having angioedema is higher is you have a history of angioedema.
What are the most common side effects of Cryselle?
- menstrual complaints, including unscheduled bleeding and spotting
- nausea
- headache (including migraine)
- weight increase or decrease
- cervical erosion
- acne
- menstrual cramps
- vaginal discharge
- stomach pain, discomfort, and gas
- increase in appetite
- depression
- nervousness
- dark areas on your face
- fatigue
- worsening of varicose veins
These are not all the possible side effects of Cryselle. For more information, ask your healthcare provider or pharmacist. You may report side effects to the FDA at 1-800-FDA-1088.
What else should I know about taking Cryselle?
- If you are scheduled for any lab tests, tell your healthcare provider you are taking Cryselle. Certain blood tests may be affected by Cryselle.
- Cryselle does not protect against HIV infection (AIDS) and other sexually transmitted infections.
How should I store Cryselle?
- Store Cryselle at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Cryselle and all medicines out of the reach of children.
- Store away from light.
General information about the safe and effective use of Cryselle:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Cryselle for a condition for which it was not prescribed. Do not give Cryselle to other people, even if they have the same symptoms that you have. This Patient Information summarizes the most important information about Cryselle. You can ask your pharmacist or healthcare provider for information about Cryselle that is written for health professionals. For more information, call 1-888-838-2872.
Do birth control pills cause cancer?
Birth control pills do not seem to cause breast cancer. However, if you have breast cancer now, or have had it in the past, do not use birth control pills because some breast cancers are sensitive to hormones.
Women who use birth control pills may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What if I want to become pregnant?
You may stop taking the pill whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking the pill.
What should I know about my period when taking Cryselle?
Your periods may be lighter and shorter than usual. Some women may miss a period. Irregular vaginal bleeding or spotting may happen while you are taking Cryselle, especially during the first few months of use. This usually is not a serious problem. It is important to continue taking your pills on a regular schedule to prevent a pregnancy.
What are the ingredients in Cryselle?
Active ingredients:
Each white pill contains norgestrel and ethinyl estradiol.
Inactive ingredients:
White pills: hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol and pregelatinized corn starch.
Light-green pills: D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake and FD&C Yellow No. 6 Aluminum Lake.
Instructions for Use
Important Information about taking Cryselle
- Take 1 pill every day at the same time. Take the pills in the order directed on your pill dispenser.
- Do not skip your pills, even if you do not have sex often. If you miss pills (including starting the pack late) you could get pregnant. The more pills you miss, the more likely you are to get pregnant.
- If you have trouble remembering to take Cryselle, talk to your healthcare provider.
- When you first start taking Cryselle, spotting or light bleeding in between your periods may occur. Contact your healthcare provider if this does not go away after a few months.
- You may feel sick to your stomach (nauseous), especially during the first few months of taking Cryselle. If you feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If your nausea does not go away, call your healthcare provider.
- Missing pills can also cause spotting or light bleeding, even when you take the missed pills later. On the days you take 2 pills to make up for missed pills (see WHAT TO DO IF YOU MISS PILLS? below), you could also feel a little sick to your stomach.
- It is not uncommon to miss a period. However, if you miss a period and have not taken Cryselle according to directions, or miss 2 periods in a row, or feel like you may be pregnant, call your healthcare provider. If you have a positive pregnancy test, you should stop taking Cryselle.
- If you have vomiting or diarrhea within 3 to 4 hours of taking your pill, take another pill of the same color from your extra pill dispenser. If you do not have an extra pill dispenser, take the next pill in the dispenser you are currently using. Continue taking all your remaining pills in order. Start the first pill of your next pill dispenser the day after finishing your current pill dispenser. This will be 1 day earlier than originally scheduled. Continue on your new schedule.
- If you have vomiting or diarrhea for more than 1 day, your birth control pills may not work as well. Use an additional birth control method, like condoms, until you check with your healthcare provider.
- Stop taking Cryselle at least 4 weeks before you have major surgery and do not restart after the surgery without asking your healthcare provider. Be sure to use another form of contraception (like condoms or spermicide) during this time period.
BEFORE YOU START TAKING CRYSELLE
- DECIDE WHAT TIME OF DAY YOU WANT TO TAKE YOUR PILL. It is important to take it at about the same time every day.
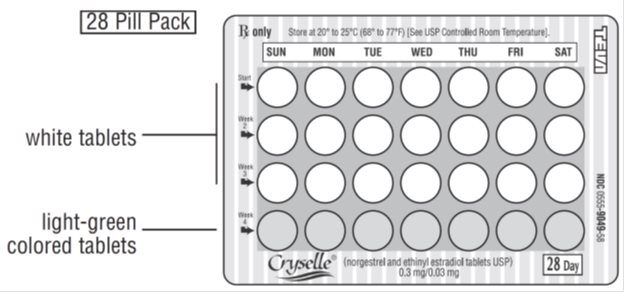
- LOOK AT YOUR PILL PACK:
The pill pack has 21 "active" white pills (with hormones) to take for 3 weeks, followed by 1 week of reminder light-green pills (without hormones). - FIND:
1) where on the pack to start taking pills, and
2) in what order to take the pills (follow the arrows) and
3) the week numbers printed on the pack.
4. BE SURE YOU HAVE READY AT ALL TIMES:
- Another kind of birth control (such as condoms or spermicide) to use as a back-up in case you miss pills
- An extra, full pill pack
WHEN TO START THE FIRST PACK OF PILLS
You have a choice of which day to start taking your first pack of pills. (See DAY 1 START or SUNDAY START directions below.) Decide with your doctor or clinic which is the best day for you. Pick a time of day which will be easy to remember.DAY 1 START:
- Pick the day label strip that starts with the first day of your period (this is the day you start bleeding or spotting, even if it is almost midnight when the bleeding begins.)
- Place this day label strip on the cycle tablet dispenser over the area that has the days of the week (starting with Sunday) printed on the blister card.
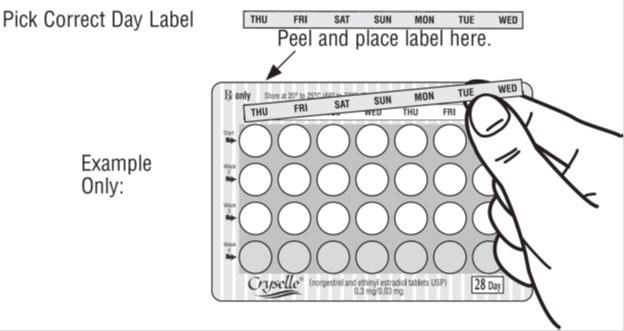
Note: If the first day of your period is a Sunday, you can skip steps #1 and #2.
3. Take the first "active" [white] pill of the first pack during the first 24 hours of your period.
4. You will not need to use a back-up method of birth control, since you are starting the pill at the beginning of your period.
SUNDAY START:
- Take the first "active" white pill of the first pack on the Sunday after your period starts, even if you are still bleeding. If your period begins on Sunday, start the pack that same day.
- Use a non-hormonal method of birth control (such as condoms or spermicide) as a back-up method if you have sex anytime from the Sunday you start your first pack until the next Sunday (7 days).
WHAT TO DO DURING THE MONTH
- Take one pill at the same time every day until the pack is empty.
Do not skip pills even if you are spotting or bleeding between monthly periods or feel sick to your stomach (nausea).
Do not skip pills even if you do not have sex very often. - When you finish a pack:
Start the next pack on the day after your last "reminder" pill. Do not wait any days between packs.
IF YOU SWITCH FROM ANOTHER BRAND OF COMBINATION PILLS:
If your previous brand had 21 pills: Wait 7 days to start taking Cryselle. You will probably have your period during that week. Ideally, be sure that no more than 7 days pass between the 21-day pack and taking the first white Cryselle pill (“active” with hormone). If you start Cryselle more than 7 days after taking the last pill of your previous contraceptive, you must use a non-hormonal back-up method of birth control during the first 7 days of Cryselle use.
If your previous brand had 28 pills: Start taking the first white Cryselle pill (“active” with hormone) on the day after your last reminder pill. Ideally, do not wait any days between packs. If you do skip any days between the last pill of your previous contraceptive and starting Cryselle, you must use a non-hormonal back-up method of birth control during the first 7 days of Cryselle use.
IF YOU SWITCH FROM ANOTHER TYPE OF BIRTH CONTROL METHOD:
If you were previously taking a progestin-only PILL: You may switch to Cryselle on any day from a progestin-only pill and should start taking the first white Cryselle pill (“active” with hormone) the day after you take your last progestin-only pill. In addition, use a non-hormonal back-up method of birth control for the first 7 days of tablet-taking.
If you are switching from a contraceptive vaginal ring or transdermal patch: Start taking the first white Cryselle pill (“active” with hormone) on the day that you would have inserted a new ring or applied a new patch.
If you are switching from a contraceptive implant: Start taking the first white Cryselle pill (“active” with hormone) on the day that the implant is removed.
If you are switching from a contraceptive injection: Start taking the first white Cryselle pill (“active” with hormone) on the day that the next contraceptive injection is due.
If you are switching from an Intrauterine device (IUD) or Intrauterine system (IUS): Start taking the first white Cryselle pill (“active” with hormone) on the day the IUD/IUS is removed. If your IUD/IUS is removed on the first day of your period you do not need to use an additional non-hormonal back up method of birth control. If the IUD/IUS is removed on any other day, use a non-hormonal back-up method of birth control for the first 7 days of tablet-taking.
WHAT TO DO IF YOU MISS PILLS
Cryselle may not be as effective if you miss white “active” pills, and particularly if you miss the first few or the last few white “active” pills in a pack.
If you MISS 1 white “active” pill:
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- You could become pregnant if you have sex in the 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as condoms or spermicide) as a back-up for those 7 days.
If you MISS 2 white “active” pills in a row in WEEK 1 OR WEEK 2 of your pack:
- Take 2 pills on the day you remember and 2 pills the next day.
- Then take 1 pill a day until you finish the pack.
- You could become pregnant if you have sex in the 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as condoms or spermicide) as a back-up for those 7 days.
If you MISS 2 white “active” pills in a row in THE 3rd WEEK:
- Keep taking 1 pill every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of pills that same day.
- You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your healthcare provider because you might be pregnant.
- You could become pregnant if you have sex in the 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as condoms or spermicide) as a back-up for those 7 days.
If you MISS 3 OR MORE white “active” pills in a row (during the first 3 weeks):
- Keep taking 1 pill every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of pills that same day.
- You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your healthcare provider because you might be pregnant.
- You could become pregnant if you have sex in the 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as condoms or spermicide) as a back-up for those 7 days.
If you forget any of the 7 light-green “reminder” pills in Week 4:
Throw away the pills you missed. Keep taking 1 pill each day until the pack is empty. You do not need a back-up non-hormonal birth control method if you start your next pack on time.
FINALLY, IF YOU ARE STILL NOT SURE WHAT TO DO ABOUT THE PILLS YOU HAVE MISSED
Use a back-up non-hormonal birth control method anytime you have sex.
Keep taking one pill each day until you can reach your healthcare provider.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Rev. C 12/2017
- Package/Label Display Panel, Part 1 of 2
-
Package/Label Display Panel, Part 2 of 2

Cryselle® (norgestrel and ethinyl estradiol tablets USP) Kit, 6 Blister Card Dispensers – 28 Tablets Each, Carton Text
NDC 0555-9049-58
6 Blister Card Dispensers, 28 Tablets Each
Cryselle®
(norgestrel and
ethinyl estradiol tablets USP)
0.3 mg/0.03 mg
21 white tablets, each containing 0.3 mg norgestrel, USP with 0.03 mg
ethinyl estradiol, USP, and 7 light-green inert tablets.
Rx only
SHAPING
WOMEN’S HEALTH®
TEVA
-
INGREDIENTS AND APPEARANCE
CRYSELLE
norgestrel and ethinyl estradiol kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0555-9049 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0555-9049-58 6 in 1 CARTON 07/24/2002 1 NDC: 0555-9049-79 1 in 1 POUCH 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 21 Part 2 7 Part 1 of 2 NORGESTREL AND ETHINYL ESTRADIOL
norgestrel and ethinyl estradiol tablet, film coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NORGESTREL (UNII: 3J8Q1747Z2) (NORGESTREL - UNII:3J8Q1747Z2) NORGESTREL 0.3 mg ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 0.03 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code dp;543 Contains 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075840 07/24/2002 Part 2 of 2 INERT
inert tabletProduct Information Route of Administration ORAL Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color GREEN (light-green) Score no score Shape ROUND Size 6mm Flavor Imprint Code dp;331 Contains 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075840 07/24/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075840 07/24/2002 Labeler - Teva Pharmaceuticals USA, Inc. (001627975)
Trademark Results [Cryselle]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CRYSELLE 76163782 2707617 Live/Registered |
TEVA WOMEN'S HEALTH, LLC 2000-11-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
