UREA by ACELLA PHARMACEUTICALS UREA emulsion
UREA by
Drug Labeling and Warnings
UREA by is a Prescription medication manufactured, distributed, or labeled by ACELLA PHARMACEUTICALS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
DESCRIPTION: Each gram of 50% Urea Emulsion contains 50% urea in a formulation consisting of: caprylic/capric triglyceride, cetyl alcohol, disodium EDTA, glycerin, hydroxyethyl cellulose, lactic acid, linoleic acid, PEG-6, polysorbate 60, propylene glycol, sorbitan stearate, titanium dioxide, triethanolamine, purified water, vitamin E, xanthan gum and zinc undecylenate.
Urea is a diamide of carbonic acid with the following chemical structure:
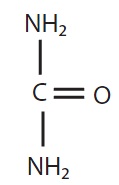
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
-
INDICATIONS & USAGE
INDICATIONS AND USAGE: For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses.
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
-
PREGNANCY
PREGNANCY: Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus; however, there are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, 50% Urea Emulsion should be given to a pregnant woman only if clearly needed.
- NURSING MOTHERS
- KEEP OUT OF REACH OF CHILDREN
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
HOW SUPPLIED: 50% Urea Emulsion is supplied in a 10 oz. tube (NDC: 42192-101-10). Store at controlled room temperature, 15° - 30° (59° - 86°F). Protect from freezing.
-
DESCRIPTION
All prescription substitutions using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the active ingredients, incipients, inactive ingredients and chemical formulation information provided herein.
MANUFACTURED FOR: Acella Pharmaceuticals, LLC
9005 Westside Parkway
Alpharetta, GA 30009
1-800-541-4802 -
DOSAGE & ADMINISTRATION
How to properly use
50% Urea
Emulsion
In a zinc undecylenate and lactic acid vehicle
Easy steps to treat dry skin conditions including psoriasis, xerosis, ichthyosis, keratosis pilaris, keratosis palmaris,
keratoderma, dermatitis, pruritus, eczema, corns and calluses.
For skin:
1. Apply 50% Urea Emulsion to affected skin twice per day, or as directed by a physician.
2. Rub in until completely absorbed (for best results apply to moistened skin).
-
PRINCIPAL DISPLAY PANEL
NDC: 42192-101-10
50% Urea
Emulsion
In a zinc undecylenate and lactic acid vehicleRx Only
Net Weight 10 oz
For Topical Use OnlyIndications: For use on rough dry skin
conditions. See carton or package insert for
instructions.USE ONLY AS DIRECTED BY A
PHYSICIAN.Ingredients: Each gram of 50% Urea
Emulsion contains 50% urea in a formulation
consisting of caprylic or capric triglyceride,
cetyl alcohol, disodium EDTA, glycerin,
hydroxyethyl cellulose, lactic acid, linoleic
acid, PEG-6, polysorbate 60, propylene
glycol, sorbitan stearate, titanium dioxide,
triethanolamine, purified water, vitamin E,
xanthan gum and zinc undecylenate.KEEP THIS AND ALL MEDICATIONS OUT
OF THE REACH OF CHILDREN.
FOR EXTERNAL USE ONLY.
NOT FOR OPHTHALMIC USE.Storage: Store at controlled room temperature
15 - 30C (59 - 86F) Protect from
freezing.For lot number and expiration date, see
crimp of tube.All prescription substitutions using this
product shall be made subject to state and
federal statutes as applicable. Note: This is
not an Orange Book product. Please see
insert for further details.Manufactured for:
Acella Pharmaceuticals
9005 Westside Parkway
Alpharetta, GA 30009
1-800-541-4802
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA
urea emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42192-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 50 g in 100 g Inactive Ingredients Ingredient Name Strength TRICAPRIN (UNII: O1PB8EU98M) CETYL ALCOHOL (UNII: 936JST6JCN) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) POWDERED CELLULOSE (UNII: SMD1X3XO9M) LACTIC ACID (UNII: 33X04XA5AT) LINOLEIC ACID (UNII: 9KJL21T0QJ) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) XANTHAN GUM (UNII: TTV12P4NEE) ZINC UNDECYLENATE (UNII: 388VZ25DUR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42192-101-10 283.5 g in 1 TUBE; Type 0: Not a Combination Product 06/29/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/29/2009 Labeler - ACELLA PHARMACEUTICALS (825380939)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
