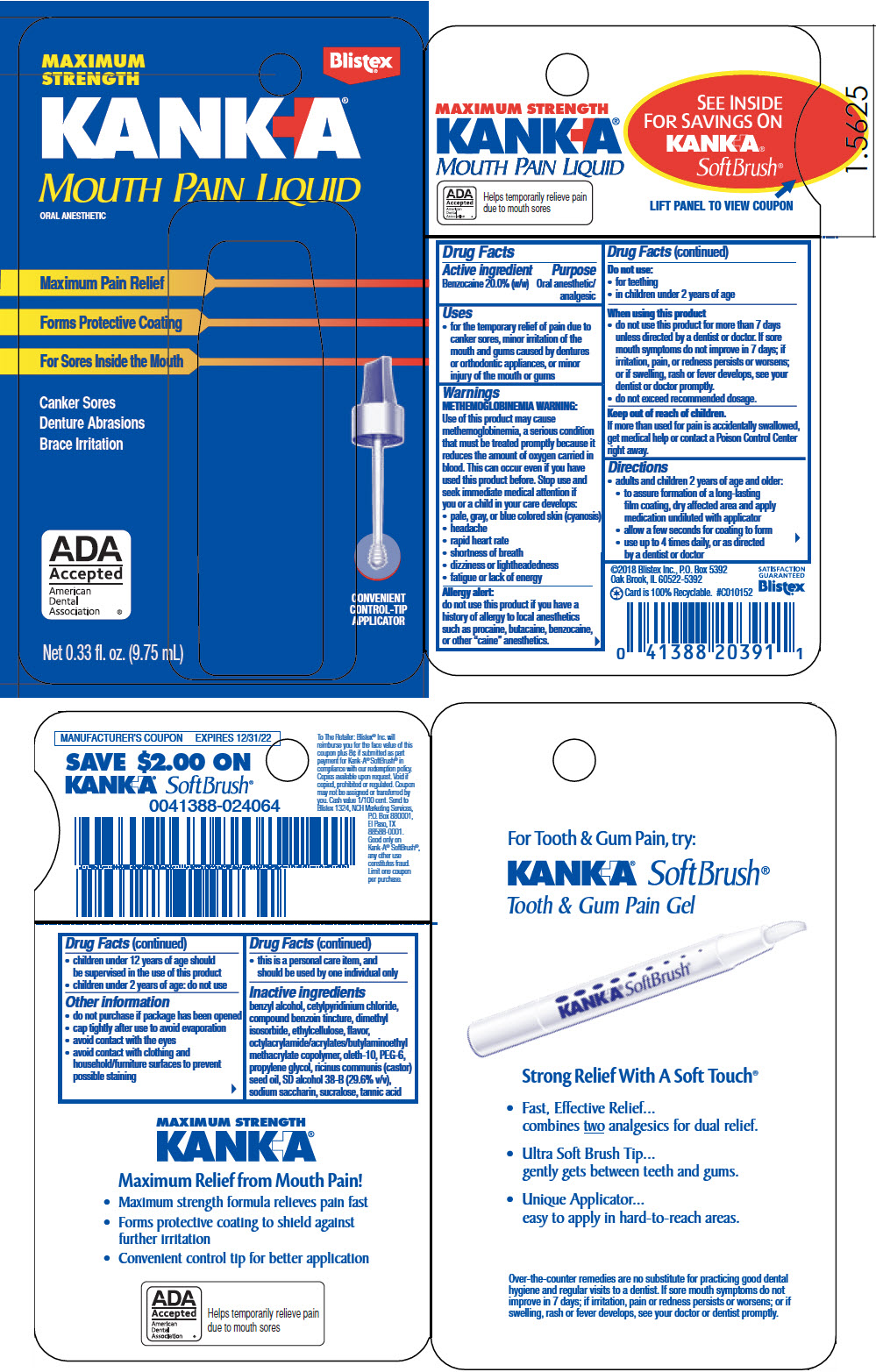
KANK-A MOUTH PAIN- benzocaine liquid
Kank-A by
Drug Labeling and Warnings
Kank-A by is a Otc medication manufactured, distributed, or labeled by Blistex Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
METHEMOGLOBINEMIA WARNING
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
When using this product
- do not use this product for more than 7 days unless directed by a dentist or doctor. If sore mouth symptoms do not improve in 7 days; if irritation, pain, or redness persists or worsens; or if swelling, rash or fever develops, see your doctor or dentist promptly.
- do not exceed recommended dosage.
-
Directions
- adults and children 2 years of age and older:
- to assure formation of a long-lasting film coating, dry affected area and apply medication undiluted with applicator
- allow a few seconds for coating to form
- use up to 4 times daily, or as directed by a dentist or doctor
- children under 12 years of age should be supervised in the use of this product
- children under 2 years of age: do not use
- adults and children 2 years of age and older:
- Other information
-
Inactive ingredients
benzyl alcohol, cetylpyridinium chloride, compound benzoin tincture, dimethyl isosorbide, ethylcellulose, flavor, octylacrylamide/acrylates/butylaminoethyl methacrylate copolymer, oleth-10, PEG-6, propylene glycol, ricinus communis (castor) seed oil, SD alcohol 38B (29.6% v/v), sodium saccharin, sucralose, tannic acid
- PRINCIPAL DISPLAY PANEL - 9.75 mL Bottle Package
-
INGREDIENTS AND APPEARANCE
KANK-A MOUTH PAIN
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10157-9477 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) POLYOXYL-10 OLEYL ETHER (UNII: JD797EF70J) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CASTOR OIL (UNII: D5340Y2I9G) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SUCRALOSE (UNII: 96K6UQ3ZD4) TANNIC ACID (UNII: 28F9E0DJY6) Product Characteristics Color ORANGE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10157-9477-1 9.75 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 12/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 12/01/2011 Labeler - Blistex Inc. (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 MANUFACTURE(10157-9477)
Trademark Results [Kank-A]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KANK-A 71589897 0539027 Live/Registered |
GEYER, JOHN A. 1949-12-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.