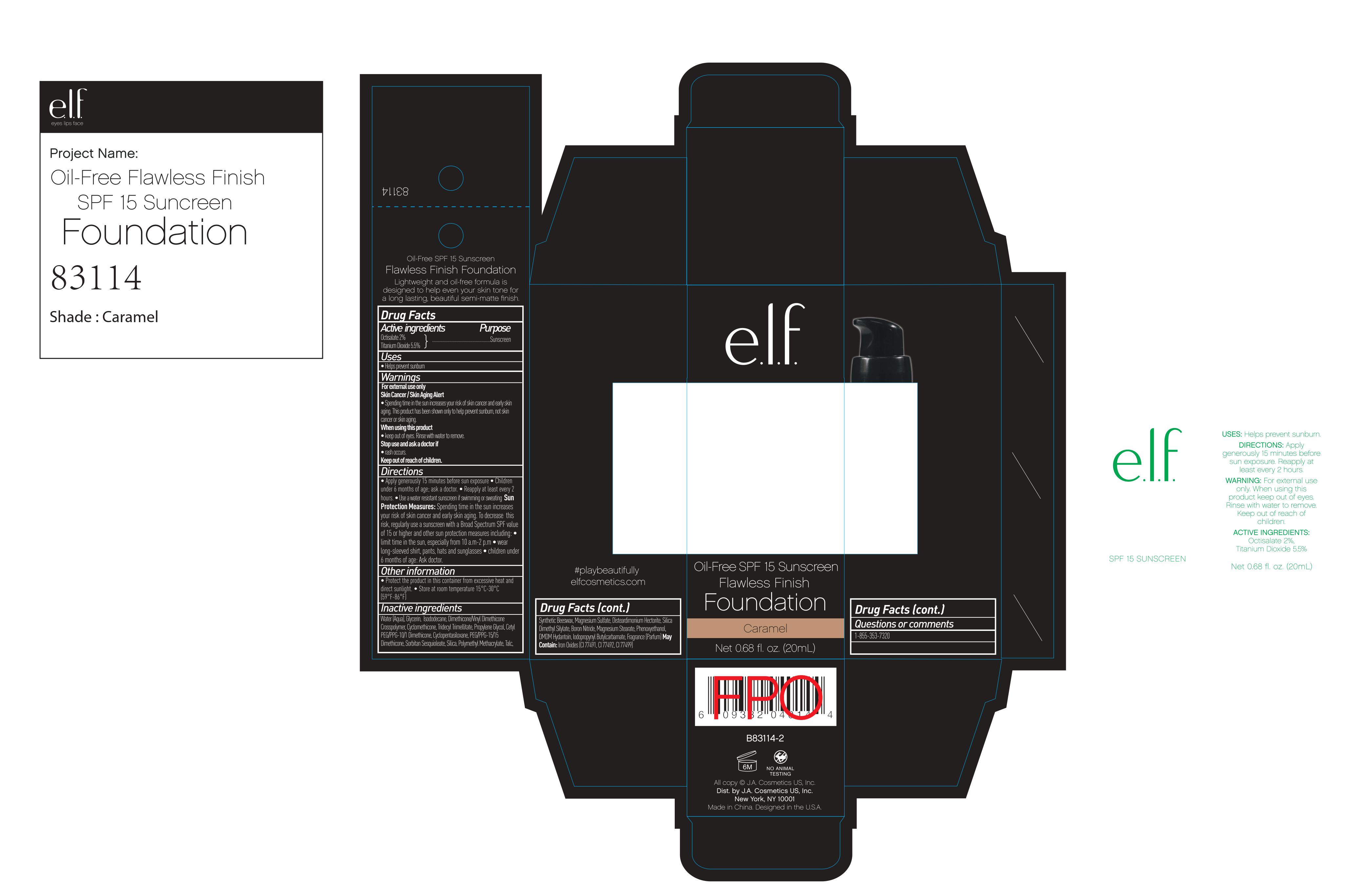
ELF Oil free flawless Finish SPF 15 Sunscreen Foundation Caramel by J. A. Cosmetics U.S. INC / Hangzhou Facecare Cosmetics Co., Ltd. Drug Fact
ELF Oil free flawless Finish SPF 15 Sunscreen Foundation Caramel by
Drug Labeling and Warnings
ELF Oil free flawless Finish SPF 15 Sunscreen Foundation Caramel by is a Otc medication manufactured, distributed, or labeled by J. A. Cosmetics U.S. INC, Hangzhou Facecare Cosmetics Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ELF OIL FREE FLAWLESS FINISH SPF 15 SUNSCREEN FOUNDATION CARAMEL- octisalate cream
J. A. Cosmetics U.S. INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Fact
Warning:
For external use only
Skin Cancer/ Skin Aging Alert: Spending time in the sun increases your risk of skin cancer or early skin aging. This product has been shown to only protect you from sunburn, not skin cancer or sking aging.
Directions:
For sunscreen use:
Apply generously 15 min before sun exposure.
Children under six months of age: ask a doctor.
Reapply at least every two hours.
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures:
Spending time in sun increases your risk of skin cancer or early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF value of 15 or higher and other sun protection measures including
- Limit time in the sun, especially from 10 am to 2 pm
- Wear long sleeved shirts, pants, hats, and sunglasses.
- Children under six months of age: ask a doctor
Other Information:
Protect this product in the container from excessive heat and direct sun
Store at room temperature 15-30 C (59-86F)
Inactive Ingredient:
Water, Dimethicone, Glycerin, Isododecane, Dimethicone/VinylDimethicone Crosspolymer, Cyclomethicone, Propylene Glycol, CETYL PEG/PPG-10/1 DIMETHICONE, Cyclopentasiloxane, Sorbitan Sesquioleate, Silica, Polymethyl Methacrylate, Talc, Synthetic Beewax, Magnesium Sulfate, Silica Dimethyl Silylate, Boron Nitride, Magnesium Stearate, Phenoxyethanol, DMDM Hydantoin, Iodopropynyl Butylcarbamate, Bentonite, Fragrance
May contain:
Iron Oxides (CI 77491, CI 77492, CI 77499)
| ELF OIL FREE FLAWLESS FINISH SPF 15 SUNSCREEN FOUNDATION CARAMEL
octisalate cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - J. A. Cosmetics U.S. INC (186705047) |