72937-026-02 72937-026-04 72937-026-08 72937-026-16
GREEN FARM POTENT COOLING RELIEF by
Drug Labeling and Warnings
GREEN FARM POTENT COOLING RELIEF by is a Otc medication manufactured, distributed, or labeled by SUNSET NOVELTIES, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
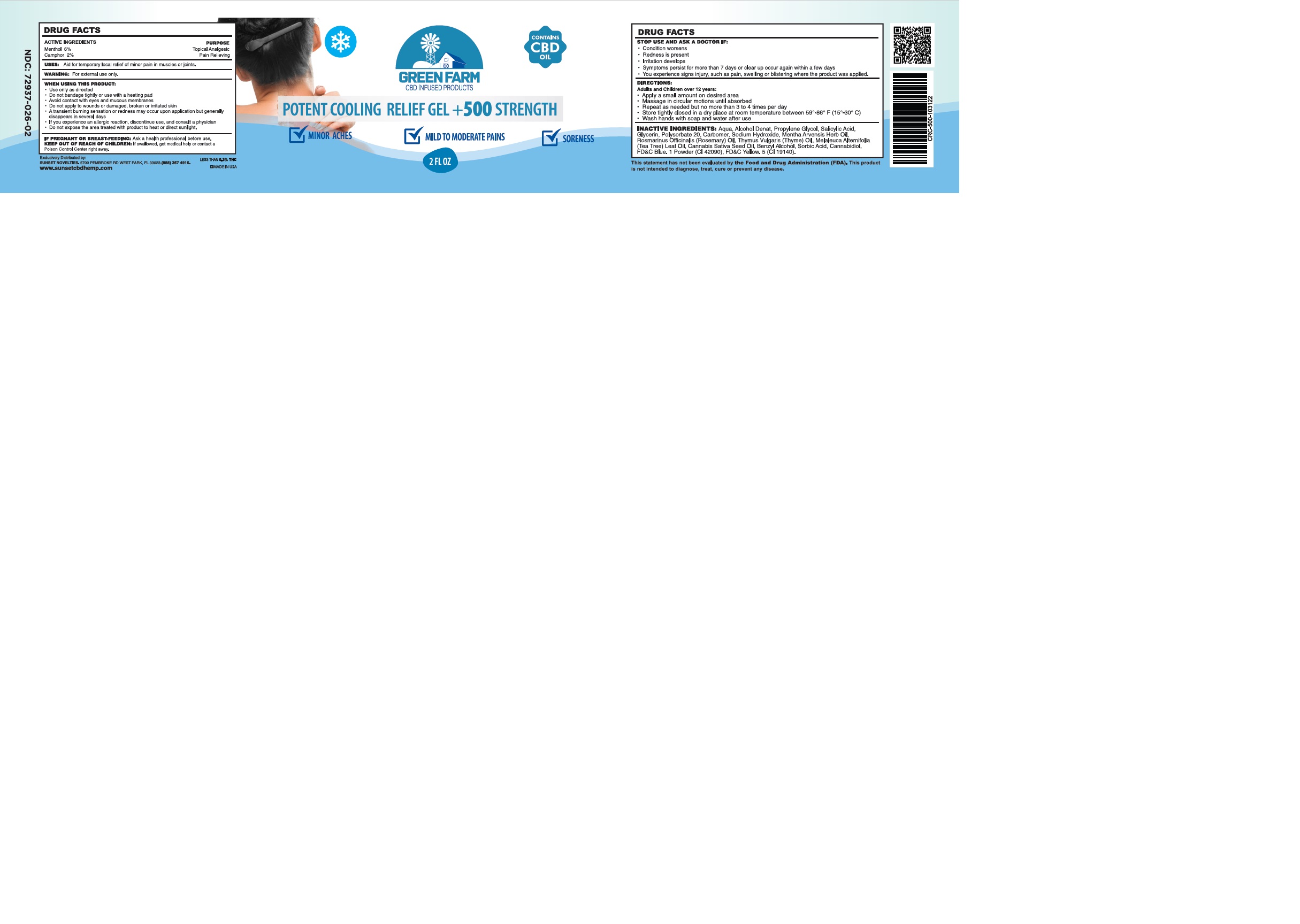
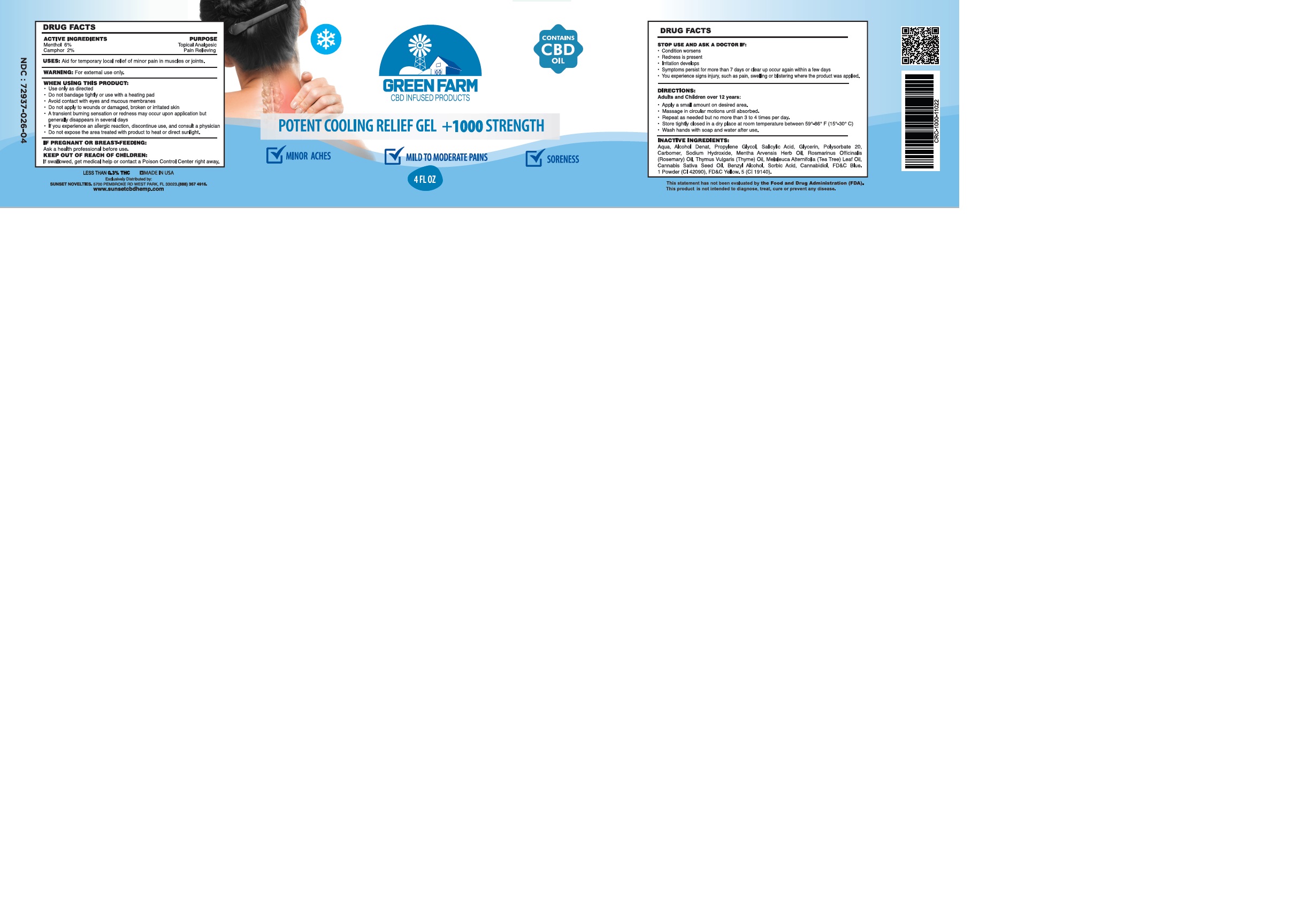
GREEN FARM POTENT COOLING RELIEF- menthol, camphor gel
SUNSET NOVELTIES, INC
----------
72937-026-02
72937-026-04
72937-026-08
72937-026-16
Use only as directed.
Do not bandage tightly or use with a heating pad.
Avoid contact with eyes and mucous membranes.
Do not apply to wounds or damaged, broken or irritated skin.
A transcient burning sensation or redness may occur upon application but generally dissapears in several days.
If you experience an allergic reaction, discontinue use, and consult a physician.
Do not expose the area treated with product to heat or direct sunlight.
STOP USE AND ASK A DOCTOR IF:
Conditions worsen
Redness is present
Irritation develops
Symptoms persist for more than 7 days or clear up occur again within a few days
You experience signs injury, such as pain, swelling or blistering where the product was applied.
DIRECTIONS:
Adults and Children over 12 years:
Apply a small amount on desired area.
Massage in circular motions until absorbed
Repeat as necessary, but no more than 3 to 4 times per day.
Store tightly closed in a dry place at controlled room temperature between 59°-86° F (15°-30° C).
Wash hands with soap and water after use.
Aqua, Alcohol Denat, Propylene Glycol, Salicylic Acid, Glycerin, Polysorbate 20, Carbomer, Sodium Hydroxide, Mentha Arvensis Herb Oil, Rosmarinus Officinalis (Rosemary) Oil, Thymus Vulgaris (Thyme) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Cannabis Sativa Seed Oil, Benzyl Alcohol, Sorbic Acid, Cannabidiol, FD&C Blue. 1 Powder (CI 42090), FD&C Yellow. 5 (CI 19140).
| GREEN FARM POTENT COOLING RELIEF
menthol, camphor gel |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - SUNSET NOVELTIES, INC (067218145) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.